Abstract
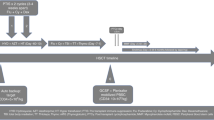
Allogeneic hematopoietic stem cell transplantation (HSCT) offers the possibility of cure for sickle cell disease (SCD) patients. Unfortunately, the probability of finding an HLA-matched donor for SCD patients is low. HSCT from HLA-haploidentical donors using reduced intensity conditioning, unmanipulated bone marrow and post-transplantation cyclophosphamide (ptCy) has resulted in negligible toxicity but high rates of graft rejection. We hypothesized that combining ptCy with a myeloablative reduced toxicity conditioning including serotherapy to increase immune ablation would allow for better engraftment. In a pilot approach, we treated three patients with SCD (5, 8, and 20 years old) lacking a matched donor. All patients had severe disease-related complications despite standard treatment. They received unmanipulated bone marrow from parental HLA-haploidentical donors. Conditioning consisted of alemtuzumab 0.2 mg/kg/day on days −9 and −8, fludarabine 30 mg/m2/day on days −7 to −3, treosulfan 14 g/m2/day on days −7 to −5, thiotepa 2 × 5 mg/kg/day on day −4, and cyclophosphamide 14.5 mg/kg/day on days −3 and −2. GVHD prophylaxis was performed using cyclophosphamide 2 × 50 mg/kg on days +3 and +4 and mycophenolate mofetil, tacrolimus from day +5. After a follow-up of 11, 14, and 30 months, all three patients are alive and well, off immunosuppression, and without symptoms of SCD. One patient experienced mild skin GVHD grade I, none showed chronic GVHD. Asymptomatic CMV reactivation was seen in two patients. HLA-haploidentical HSCT can extend the donor pool for patients with SCD. Whether intensification of the conditioning regimen and intensive immunosuppression leads to improvement in engraftment rates while still allowing a favorable toxicity profile deserves further investigation.

Similar content being viewed by others
References
Parise LV, Berliner N (2016) Sickle cell disease: challenges and progress. Blood 127(7):789. doi:10.1182/blood-2015-12-674606
Rees DC, Williams TN, Gladwin MT (2010) Sickle-cell disease. Lancet 376(9757):2018–2031. doi:10.1016/S0140-6736(10)61029-X
DeBaun MR, Gordon M, McKinstry RC, Noetzel MJ, White DA, Sarnaik SA, Meier ER, Howard TH, Majumdar S, Inusa BP, Telfer PT, Kirby-Allen M, McCavit TL, Kamdem A, Airewele G, Woods GM, Berman B, Panepinto JA, Fuh BR, Kwiatkowski JL, King AA, Fixler JM, Rhodes MM, Thompson AA, Heiny ME, Redding-Lallinger RC, Kirkham FJ, Dixon N, Gonzalez CE, Kalinyak KA, Quinn CT, Strouse JJ, Miller JP, Lehmann H, Kraut MA, Ball WS Jr, Hirtz D, Casella JF (2014) Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J med 371(8):699–710. doi:10.1056/NEJMoa1401731
Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW, Rother RP (2016) Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J med. doi:10.1056/NEJMoa1611770
Hoban MD, Orkin SH, Bauer DE (2016) Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease. Blood 127(7):839–848. doi:10.1182/blood-2015-09-618587
Bhatia M, Kolva E, Cimini L, Jin Z, Satwani P, Savone M, George D, Garvin J, Paz ML, Briamonte C, Cruz-Arrieta E, Sands S (2015) Health-related quality of life after allogeneic hematopoietic stem cell transplantation for sickle cell disease. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 21(4):666–672. doi:10.1016/j.bbmt.2014.12.007
Locatelli F, Pagliara D (2012) Allogeneic hematopoietic stem cell transplantation in children with sickle cell disease. Pediatr Blood Cancer 59(2):372–376. doi:10.1002/pbc.24177
Bernaudin F, Socie G, Kuentz M, Chevret S, Duval M, Bertrand Y, Vannier JP, Yakouben K, Thuret I, Bordigoni P, Fischer A, Lutz P, Stephan JL, Dhedin N, Plouvier E, Margueritte G, Bories D, Verlhac S, Esperou H, Coic L, Vernant JP, Gluckman E, Sfgm TC (2007) Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood 110(7):2749–2756. doi:10.1182/blood-2007-03-079665
Gluckman E, Cappelli B, Bernaudin F, Labopin M, Volt F, Carreras J, Simoes BP, Ferster A, Dupont S, de la Fuente J, Dalle JH, Zecca M, Walters MC, Krishnamurti L, Bhatia M, Leung K, Yanik G, Kurtzberg J, Dhedin N, Kuentz M, Michel G, Apperley J, Lutz P, Neven B, Bertrand Y, Vannier JP, Ayas M, Cavazzana M, Matthes-Martin S, Rocha V, Elayoubi H, Kenzey C, Bader P, Locatelli F, Ruggeri A, Eapen M (2016) Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. doi:10.1182/blood-2016-10-745711
Shenoy S (2013) Hematopoietic stem-cell transplantation for sickle cell disease: current evidence and opinions. Therapeutic advances in hematology 4(5):335–344. doi:10.1177/2040620713483063
Justus D, Perez-Albuerne E, Dioguardi J, Jacobsohn D, Abraham A (2015) Allogeneic donor availability for hematopoietic stem cell transplantation in children with sickle cell disease. Pediatr Blood Cancer. doi:10.1002/pbc.25439
Shenoy S, Eapen M, Panepinto JA, Logan BR, Wu J, Abraham A, Brochstein J, Chaudhury S, Godder K, Haight AE, Kasow KA, Leung K, Andreansky M, Bhatia M, Dalal J, Haines H, Jaroscak J, Lazarus HM, Levine JE, Krishnamurti L, Margolis D, Megason GC, Yu LC, Pulsipher MA, Gersten I, DiFronzo N, Horowitz MM, Walters MC, Kamani N (2016) A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood 128(21):2561–2567. doi:10.1182/blood-2016-05-715870
Tolar J, Sodani P, Symons H (2015) Alternative donor transplant of benign primary hematologic disorders. Bone marrow transplant 50(5):619–627. doi:10.1038/bmt.2015.1
Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, Brodsky RA (2012) HLA-haploidentical bone marrow transplantation with post-transplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood 120(22):4285–4291. doi:10.1182/blood-2012-07-438408
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 11(12):945–956. doi:10.1016/j.bbmt.2005.09.004
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 15(6):825–828
Kamani NR, Walters MC, Carter S, Aquino V, Brochstein JA, Chaudhury S, Eapen M, Freed BM, Grimley M, Levine JE, Logan B, Moore T, Panepinto J, Parikh S, Pulsipher MA, Sande J, Schultz KR, Spellman S, Shenoy S (2012) Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the phase II study from the blood and marrow transplant clinical trials network (BMT CTN). Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 18(8):1265–1272. doi:10.1016/j.bbmt.2012.01.019
Bayraktar UD, Champlin RE, Ciurea SO (2012) Progress in haploidentical stem cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 18(3):372–380. doi:10.1016/j.bbmt.2011.08.001
Kanakry CG, Fuchs EJ, Luznik L (2016) Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat rev Clin Oncol 13(1):10–24. doi:10.1038/nrclinonc.2015.128
Dallas MH, Triplett B, Shook DR, Hartford C, Srinivasan A, Laver J, Ware R, Leung W (2013) Long-term outcome and evaluation of organ function in pediatric patients undergoing haploidentical and matched related hematopoietic cell transplantation for sickle cell disease. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 19(5):820–830. doi:10.1016/j.bbmt.2013.02.010
Marzollo A, Calore E, Tumino M, Pillon M, Gazzola MV, Destro R, Colombatti R, Marson P, Tison T, Colpo A, Mainardi C, Gabelli M, Boaro MP, Rossin S, Strano A, Quaglia N, Menzato F, Basso G, Sainati L, Messina C (2017) Treosulfan-based conditioning regimen in sibling and alternative donor hematopoietic stem cell transplantation for children with sickle cell disease. Mediterr J Hematol Infect Dis 9(1):e2017014. doi:10.4084/MJHID.2017.014
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, Morris LE, Solomon SR (2013) T cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology 31(10):1310–1316. doi:10.1200/JCO.2012.44.3523
Anurathapan U, Hongeng S, Pakakasama S, Sirachainan N, Songdej D, Chuansumrit A, Charoenkwan P, Jetsrisuparb A, Sanpakit K, Rujkijyanont P, Meekaewkunchorn A, Lektrakul Y, Iamsirirak P, Surapolchai P, Satayasai W, Sirireung S, Sruamsiri R, Wahidiyat PA, Ungkanont A, Issaragrisil S, Andersson BS (2016) Hematopoietic stem cell transplantation for homozygous beta-thalassemia and beta-thalassemia/hemoglobin E patients from haploidentical donors. Bone Marrow Transplant 51(6):813–818. doi:10.1038/bmt.2016.7
Bernardo ME, Piras E, Vacca A, Giorgiani G, Zecca M, Bertaina A, Pagliara D, Contoli B, Pinto RM, Caocci G, Mastronuzzi A, La Nasa G, Locatelli F (2012) Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood 120(2):473–476. doi:10.1182/blood-2012-04-423822
Strocchio L, Zecca M, Comoli P, Mina T, Giorgiani G, Giraldi E, Vinti L, Merli P, Regazzi M, Locatelli F (2015) Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in children with sickle cell disease. Br J Haematol 169(5):726–736. doi:10.1111/bjh.13352
Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, Bregante S, Van Lint MT, Varaldo R, Ghiso A, Gobbi M, Carella AM, Signori A, Galaverna F, Bacigalupo A (2014) Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation 20(10):1573–1579. doi:10.1016/j.bbmt.2014.05.029
Author information
Authors and Affiliations
Contributions
All authors were involved in the clinical care of the patients. VW and MHA wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Patients or their respective caregivers gave their written informed consent to treatment and for inclusion in the German Pediatric Stem Cell Transplantation Registry (PRST), which was approved by the ethics committee of the Medizinische Hochschule Hannover.
Rights and permissions
About this article
Cite this article
Wiebking, V., Hütker, S., Schmid, I. et al. Reduced toxicity, myeloablative HLA-haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for sickle cell disease. Ann Hematol 96, 1373–1377 (2017). https://doi.org/10.1007/s00277-017-3030-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3030-x