Abstract
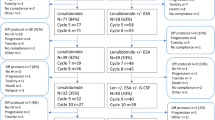
Transfusion-dependent patients with low- or intermediate-1-risk myelodysplastic syndrome, <5% bone marrow (BM) blasts and isolated 5q-deletion received lenalidomide within the German MDS study group phase-II clinical trial LE-MON-5 (EudraCT:2008-001866-10) of the University of Duesseldorf, Germany. Cytogenetic monitoring was performed by chromosome banding analyses (CBA) of BM cells and fluorescence in situ hybridization (FISH) analyses of peripheral blood (PB) mononuclear CD34+ cells using extended probe panels. Out of 144 patients screened for study enrollment, 24% failed to meet inclusion criteria due to cytogenetic findings. Eighty-seven patients were followed with a median observation time of 30 months. Cytogenetic response detected by FISH and CBA in 74 and 66% of patients, respectively, was predictive for hematologic response as well as of high prognostic relevance. After 2 years, AML rate was 8% for all patients. Karyotype evolution was detected in 21 (FISH)–34% (CBA) of patients associated with significantly shorter AML-free survival. Disease progression was first detectable on the cytogenetic level on average 5–6 months before recurrence of transfusion dependence. Our data show for the first time in a prospective setting that a cytogenetic monitoring from the PB helps to early identify treatment failure and progressive disease in lenalidomide-treated patients to improve clinical management. Trial registration: EudraCT:2008-001866-10


Similar content being viewed by others
References
Greenberg P, Cox C, LeBeau MM et al (1997) International scoring system for evaluating prognosis in myelodysplstic syndromes. Blood 89:2079–2088
Greenberg PL, Tuechler H, Schanz J et al (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120(12):2454–2465
Schanz J, Tüchler H, Solé F et al (2012) New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol 30(8):820–829
Haase D, Germing U, Schanz J et al (2007) New insights into prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 110:4385–4395
List A, Kurtin S, Roe DJ et al (2005) Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 352:549–557
List A, Dewald G, Bennett J et al (2006) Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 355:1456–1465
List AF, Bennett JM, Sekeres MA et al (2014) Extended survival and reduced risk of AML progression in erythroid-responsive lenalidomide-treated patients with lower-risk del(5q) MDS. Leukemia 28:1033–1040
Arber DA, Orazi A, Hasserjian R et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405
Fenaux P, Giagounidis A, Selleslag D et al (2011) A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low−/intermediate-1-risk myelodysplastic syndromes with del5q. Blood 118(14):3765–3776
Braulke F, Schanz J, Jung K et al (2010) FISH analysis of circulating CD34+ cells as a new tool for genetic monitoring in MDS: verification of the method and application to 27 MDS patients. Leuk Res 34:1296–1301
Braulke F, Jung K, Schanz J et al (2013) Molecular cytogenetic monitoring from CD34+ peripheral blood cells in myelodysplastic syndromes: first results from a prospective multicenter German diagnostic study. Leuk Res 37:900–906
Braulke F, Platzbecker U, Muller-Thomas C et al (2015) Validation of cytogenetic risk groups according to International Prognostic Scoring Systems by peripheral blood CD34+FISH: results from a German diagnostic study in comparison with an international control group. Haematologica 100:205–213
Schuler E, Giagounidis A, Haase D et al (2015) Results of a multicenter prospective phase II trial investigating the safety and efficacy of lenalidomide in patients with myelodysplastic syndromes with isolated del(5q) (LE-MON 5). Leukemia 30(7):1580–1582
Haase D, Feuring-Buske M, Konemann S et al (1995) Evidence for malignant transformation in acute myeloid leukemia at the level of early hematopoietic stem cells by cytogenetic analysis of CD34+ subpopulations. Blood 86:2906–2912
Shaffer LG, McGowan-Jordan J, Schmid M (eds) (2013) An international system for human cytogenetic nomenclature: recommendations of the international standing committee on human cytogenetic nomenclature. S. Karger A.G, Basel
Cheson BD, Bennett JM, Kantarjian H et al (2000) World Health Organization (WHO) international working group. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 96(12):3671–3674
Chesnais V, Renneville A, Toma A et al (2016) Effect of lenalidomide treatment on clonal architecture of myelodysplastic syndromes without 5q deletion. Blood 127(6):749–760
Giagounidis A, Mufti GJ, Mittelman M et al (2014) Outcomes in RBC transfusion-dependent patients with low−/intermediate-1-risk myelodysplastic syndromes with isolated deletion 5q treated with lenalidomide: a subset analysis from the MDS-004 study. Eur J Haematol 93(5):429–438
Mallo M, Cervera J, Schanz J et al (2011) Impact of adjunct cytogenetic abnormalities for prognostic stratification in patients with myelodysplastic syndrome and deletion 5q. Leukemia 25(1):110–120
Rollison DE, Shain KH, Lee J-H et al (2016) Subsequent primary malignancies and acute myelogeneous leukemia transformation among myelodysplastic syndrome patients treated with or without lenalidomide. Cancer Med 5(7):1694–1701
Tehranchi R, Woll PS, Anderson K et al (2010) Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med 363(11):1025–1037
Mossner M, Jann J-C, Wittig J et al (2016) Mutational hierarchies in myelodysplastic syndromes dynamically adapt and evolve upon therapy response and failure. Blood 128(9):1246–1259
Mossner M, Jann JC, Nowak D et al (2016) Prevalence, clonal dynamics and clinical impact of TP53 mutations in patients with myelodysplastic syndrome with isolated deletion (5q) treated with lenalidomide: results from a prospective multi-center study of the German MDS study group (GMDS). Leukemia 30(9):1956–1959
Jadersten M, Saft A, Smith A et al (2011) TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol 29(15):1971–1979
Acknowledgments
The trial was supported by Celgene, providing the compound, supporting data monitoring, and technical processing of bone marrow biopsies. FB, UG, ES, UP, FN, WKH, A Giagounidis, KG, ML, RFS, JS, UB, A Ganser, AL, PS, GB, THB, RH, LT, and DH included patients, analyzed the data, wrote the paper, and approved the final manuscript. AG performed centralized cytology. GB performed centralized pathology. DH, FB, JS, UB, and KS performed central cytogenetic banding and molecular-cytogenetic analyses. XS performed the statistical analyses, wrote the paper, and gave the final approval. UG designed the clinical trial, contributed essential data, analyzed the data, and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
This trial was supported by Celgene. DH, FB, JS, and UB received speakers honoraria and research support by Celgene and Novartis. UG, UP, and FN obtained speakers honoraria and research support from Celgene. ES and KS received travel grants by Celgene. AG obtained honoraria from Celgene. KG obtained honoraria from Celgene. RFS obtained research support from Celgene. AL obtained research funding from Celgene. GB received research support, honoraria, and travel grants by Celgene. THB is a member of the Data Safety Monitoring Committee (DSMC) of this trial. PS obtained educational grants from Celgene. The remaining authors had no competing interests concerning this project.
Electronic supplementary material
Online Resource 1
(DOCX 15.7 kb)
Online Resource 2
(DOCX 16.1 kb)
Online Resource 3
(DOCX 14.2 kb)
Online Resource 4
(DOCX 32.8 kb)
Online Resource 5
(DOCX 14.9 kb)
Online Resource 6
(DOCX 17.4 kb)
Rights and permissions
About this article
Cite this article
Braulke, F., Schulz, X., Germing, U. et al. Peripheral blood cytogenetics allows treatment monitoring and early identification of treatment failure to lenalidomide in MDS patients: results of the LE-MON-5 trial. Ann Hematol 96, 887–894 (2017). https://doi.org/10.1007/s00277-017-2983-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2983-0