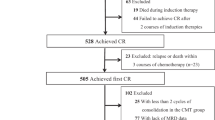
Abstract
This study investigated the efficacy of minimal residual disease (MRD) monitoring and MRD-directed preemptive immunotherapy in high-risk myelodysplastic syndrome (MDS) patients who received allogeneic hematopoietic stem cell transplantation (HSCT). MRD assessment consisted of Wilms’ tumor gene 1 (WT1) detection with PCR and leukemia-associated immunophenotypic pattern examination with multiparameter flow cytometry (FCM). Post-HSCT, 31 patients were positive for WT1, and 8, for FCM; positivity for WT1 (18.6 vs. 6.1 %, P = 0.040) or FCM (62.5 vs. 3.6 %, P < 0.001) indicated a higher 2-year relapse rate. Twenty-one patients met our combined criteria for MRD, and the presence of MRD was associated with a higher 2-year relapse rate (27.3 vs. 4.5 %, P = 0.003). Preferentially expressed antigen of melanoma (PRAME) expression alone was not an appropriate MRD marker; however, it suggested that the MRD-positive patients may fail to respond to preemptive immunotherapy. In patients positive for both PRAME and MRD, the relapse rate was 60 % despite preemptive immunotherapy. Multivariate analysis confirmed the association between the increased relapse rate and positivity for both PRAME and MRD (hazard ratio = 42.8, P = 0.001). MRD monitoring predicted relapse in high-risk MDS post-HSCT patients, and PRAME- and MRD-positive patients did not benefit from preemptive immunotherapy.



Similar content being viewed by others
References
Kindwall-Keller T, Isola LM (2009) The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant 43:597–609
Vaughn JE, Scott BL, Deeg HJ (2013) Transplantation for myelodysplastic syndromes 2013. Curr Opin Hematol 20:494–500
Yeung CC, Gerds AT, Fang M et al (2015) Relapse after allogeneic hematopoietic cell transplantation for myelodysplastic syndromes: analysis of late relapse using comparative karyotype and chromosome genome array testing. Biol Blood Marrow Transplant 21:1565–1575
Mo XD, Zhang XH, Xu LP et al (2015) Interferon-α: a potentially effective treatment for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 21:1939–1947
Steinbach D, Bader P, Willasch A et al (2015) Prospective validation of a new method of monitoring minimal residual disease in childhood acute myelogenous leukemia. Clin Cancer Res 21:1353–1359
Abdelmalak CA, Yahya RS, Elghannam DM, El-Khadragy AE, Abd El Messih HM (2014) PRAME gene expression in childhood acute lymphoblastic leukemia: impact on prognosis. Clin Lab 60:55–61
Mitsuhashi K, Masuda A, Wang YH, Shiseki M, Motoji T (2014) Prognostic significance of PRAME expression based on immunohistochemistry for diffuse large B-cell lymphoma patients treated with R-CHOP therapy. Int J Hematol 100:88–95
Ercolak V, Paydas S, Bagir E et al (2015) PRAME expression and its clinical relevance in Hodgkin’s lymphoma. Acta Haematol 134:199–207
Liberante FG, Pellagatti A, Boncheva V et al (2013) High and low, but not intermediate, PRAME expression levels are poor prognostic markers in myelodysplastic syndrome at disease presentation. Br J Haematol 162:282–285
Qin YZ, Zhu HH, Liu YR et al (2013) PRAME and WT1 transcripts constitute a good molecular marker combination for monitoring minimal residual disease in myelodysplastic syndromes. Leuk Lymphoma 54:1442–1449
Wang Y, Liu DH, Liu KY et al (2013) Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 119:978–985
Xiao-Jun H, Lan-Ping X, Kai-Yan L et al (2009) Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res 15:4777–4783
Huang XJ, Liu DH, Liu KY et al (2006) Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 38:291–297
Huang XJ, Liu DH, Liu KY et al (2009) Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 15:257–265
Mo XD, Xu LP, Liu DH et al (2013) The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) is an outcome predictor for partially matched related donor transplantation. Am J Hematol 88:497–502
Wang YZ, Liu YR, Zhu HH et al (2009) Prognostic significance of minimal residual disease detected by multiparameter flow cytometry in acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 17:551–556
Zhao XS, Liu YR, Zhu HH et al (2012) Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol 91:183–192
Zhao XS, Yan CH, Liu DH et al (2013) Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Ann Hematol 92:1111–1119
Zhao XS, Jin S, Zhu HH et al (2012) Wilms’ tumor gene 1 expression: an independent acute leukemia prognostic indicator following allogeneic hematopoietic SCT. Bone Marrow Transplant 47:499–507
Qin Y, Zhu H, Jiang B et al (2009) Expression patterns of WT1 and PRAME in acute myeloid leukemia patients and their usefulness for monitoring minimal residual disease. Leuk Res 33:384–390
Yan CH, Liu DH, Liu KY et al (2012) Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 119:3256–3262
Malcovati L, Germing U, Kuendgen A et al (2007) Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25:3503–3510
Alessandrino EP, Della Porta MG, Bacigalupo A et al (2008) WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplasticsyndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Blood 112:895–902
Gooley TA, Leisenring W, Crowley J, Storer BE (1999) Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 18:695–706
Scrucca L, Santucci A, Aversa F (2010) Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 45:1388–1395
Tamaki H, Ogawa H, Ohyashiki K et al (1999) The Wilms’ tumor gene WT1 is a good marker for diagnosis of disease progression of myelodysplastic syndromes. Leukemia 13:393–399
Yoon JH, Jeon YW, Yahng SA et al (2015) Wilms tumor gene 1 expression as a predictive marker for relapse and survival after hematopoietic stem cell transplantation for myelodysplastic syndromes. Biol Blood Marrow Transplant 21:460–467
Porwit A (2011) Role of flow cytometry in diagnostics of myelodysplastic syndromes—beyond the WHO 2008 classification. Semin Diagn Pathol 28:273–282
Dominietto A, Pozzi S, Miglino M et al (2007) Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood 109:5063–5064
Mo XD, Zhang XH, Xu LP et al (2016) Salvage chemotherapy followed by granulocyte colony-stimulating factor-primed donor leukocyte infusion with graft-vs.-host disease control for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation: prognostic factors and clinical outcomes. Eur J Haematol 96:297–308
Gesundheit B, Shapira MY, Resnick IB et al (2009) Successful cell-mediated cytokine-activated immunotherapy for relapsed acute myeloid leukemia after hematopoietic stem cell transplantation. Am J Hematol 84:188–190
Schroeder T, Czibere A, Platzbecker U et al (2013) Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 27:1229–1235
Schroeder T, Rachlis E, Bug G et al (2015) Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions—a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant 21:653–660
Mo X, Zhao X, Xu L et al (2014) Interferon α: the salvage therapy for patients with unsatisfactory response to minimal residual disease-directed modified donor lymphocyte infusion. Chin Med J (Engl) 127:2583–2587
Paydas S, Tanriverdi K, Yavuz S, Disel U, Baslamisli F, Burgut R (2005) PRAME mRNA levels in cases with acute leukemia: clinical importance and future prospects. Am J Hematol 79:257–261
Kang H, Wang X, Gao L et al (2015) Clinical implications of the quantitative detection of ID4 gene methylation in myelodysplastic syndrome. Eur J Med Res 20:16
Chatterton Z, Burke D, Emslie KR et al (2014) Validation of DNA methylation biomarkers for diagnosis of acute lymphoblastic leukemia. Clin Chem 60:995–1003
Acknowledgements
The authors thank Editage for their assistance in editing this manuscript. This work was supported by the Key Program of the National Natural Science Foundation of China (Grant 81230013), Project TG-2015-003 supported by the Health Science Promotion Project of Beijing, Beijing Talents fund (No. 2015000021223ZK39), and the National Natural Science Foundation of China (Grant 81400145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was obtained from all patients, and the study was conducted according to the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Characteristics between PRAME (+) MRD (+) and PRAME (-) MRD (+) patients. (DOC 66 kb)
Supplementary Figure 1
PRAME, MRD, and preemptive immunotherapy after HSCT. DLI: donor lymphocyte infusion; IFN: interferon; IST: immunosuppressive therapy; MRD: minimal residual disease. (TIF 5380 kb)
Rights and permissions
About this article
Cite this article
Mo, XD., Qin, YZ., Zhang, XH. et al. Minimal residual disease monitoring and preemptive immunotherapy in myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Ann Hematol 95, 1233–1240 (2016). https://doi.org/10.1007/s00277-016-2706-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2706-y