Abstract
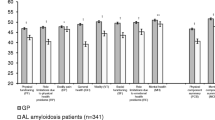
Nutritional status is an independent prognostic factor in immunoglobulin light-chain amyloidosis (AL), but its influence on quality of life (QoL) is unknown. The aim of this cross-sectional study was to investigate the association between nutritional status and QoL in AL patients at diagnosis. One hundred and fifty consecutive patients with biopsy-proven AL were assessed for nutritional status by anthropometry [body mass index, unintentional weight loss (WL) in the previous 6 months and mid-arm muscle circumference (MAMC)], biochemistry (serum prealbumin), and semiquantitative food intake at referral. QoL was assessed by the Medical Outcomes Study 36-item Short Form General Health Survey. The composite physical component summary (PCS) and the mental component summary (MCS) for AL outpatients were 36.2 ± 10.1 and 44.9 ± 11.3, respectively (p < 0.001 for both vs the population norms of 50). In multivariate linear regression models adjusted for gender, age, Eastern Cooperative Oncology Group performance status, the number of organs involved, the severity of cardiac damage, C-reactive protein, energy intake, and WL, PCS was significantly lower for serum prealbumin <200 mg/L and MAMC <10th percentile (adjusted difference 3.8, 95% CI 0.18–7.5, p = 0.040 and 5.3, 95% CI 2.0–8.7, p = 0.002, respectively). MCS was decreased by 0.47 (95% CI 0.18–0.75, p = 0.002) for each kilogram of body weight lost in the previous 6 months. Nutritional status independently affects QoL in AL patients since diagnosis. Nutritional evaluation should be integral part of the clinical assessment of AL patients. Nutritional support intervention trials are warranted in such patients' population.

Similar content being viewed by others
References
Merlini G, Stone MJ (2006) Dangerous small B-cell clones. Blood 108:2520–2530
Dispenzieri A, Gertz M, Kyle R et al (2004) Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol 22:3751–3757
Palladini G, Barassi A, Klersy C et al (2010) The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood 116:3426–3430
Caccialanza R, Palladini G, Klersy C et al (2006) Nutritional status of outpatients with systemic immunoglobulin light-chain amyloidosis (AL). Am J Clin Nutr 83:350–354
Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME (2004) Cancer: disease and nutrition are key determinants of patients' quality of life. Support Care Cancer 12:246–252
Marín Caro MM, Laviano A, Pichard C (2007) Impact of nutrition on quality of life during cancer. Curr Opin Clin Nutr Metab Care 10:480–487
Seldin DC, Anderson JJ, Sanchorawala V et al (2004) Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood 104:1888–1893
European Medicines Agency (2005) Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. Doc. ref. EMEA/CHMP/EWP/139391/2004. London (UK)
U.S. Department of Health and Human Services, Food and Drug Administration (2006) Patient-reported outcome measures: use in medical product development to support labeling claims. February
Arbustini E, Verga L, Concardi M et al (2002) Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid 9:108–114
Gertz M, Comenzo R, Falk R et al (2004) Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 79:319–328
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Quetelet A (1835) Sur l'Homme et le Developpement de ses Facultes, ou Essai des Physique Sociale (On Man and the Development of his Faculties, or Treatise on Social Physics). Bachelier, Paris
Frisancho AR (1981) New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 34:2540–2545
WHO Expert Committee (1995) The use and interpretation of anthropometry. WHO Technical Report Series n. 854. WHO, Geneva
Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study Group (2006) Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 83:1345–1350
McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD (1994) The MOS 36-item Short-Form Health Survey (SF-36), III: tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 32:40–66
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care 30:473–483
McHorney CA, Ware JE Jr, Raczek AE (1993) The MOS 36-Item Short-Form Health Survey (SF-36), II: psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31:247–263
Ware JE Jr, Kosinski M, Bayliss MS et al (1995) Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 33:AS264–AS279
Ware JE Jr, Kosinski M (2001) SF-36 physical and mental health summary scales: a manual for users of version 1, 2nd edn. QualityMetric Inc., Lincoln
Ware JE Jr, Kosinski M, Gandek B (2000) SF-36 health survey: manual and interpretation guide. QualityMetric Inc., Lincoln
Istituto Scotti Bassani (1989) Atlante Ragionato di Alimentazione. Istituto Scotti Bassani per la ricerca e l'informazione scientifica e nutrizionale, Milano
Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione (I.N.R.A.N.) (2000) Tabelle di Composizione degli Alimenti. Aggiornamento 2000. EDRA Medical Publishing and New Media
Frankenfield DC, Muth ER, Rowe WA (1998) The Harris–Benedict studies of human basal metabolism: history and limitations. J Am Diet Assoc 98:439–445
STATA Corporation (2010) STATA statistical software: release 11.1. College Station
Coates A, Gebski V, Bishop JF et al (1987) Improving the quality of life during chemotherapy for advanced breast cancer: a comparison of intermittent and continuous treatment strategies. N Engl J Med 317:1490–1495
Bishop JF, Dewar J, Toner GC et al (1999) Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol 17:2355–2364
Seidman AD, Hudis CA, Norton L (1995) Memorial Sloan-Kettering Cancer Center experience with paclitaxel in the treatment of breast cancer: from advanced disease to adjuvant therapy. Semin Oncol 22(S8):3–8
Ravasco P, Monteiro Grillo I, Camilo M (2007) Cancer wasting and quality of life react to early individualized nutritional counselling! Clin Nutr 26:7–15
Ingenbleek Y, Young V (1994) Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr 14:495–533
Vasiljevic N, Sonja Ralevic S, Marinkovic J et al (2008) The assessment of health-related quality of life in relation to the body mass index value in the urban population of Belgrade. Health Qual Life Outcomes 6:106
Guler N, Kuzu F (2009) The health-related quality of life of the health professionals working in the primary healthcare centers and its correlation with selected sociodemographic factors in Sivas, a central Anatolian city. Scientific Research and Essay 4:1547–1552
Muscaritoli M, Anker SD, Argilés J et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 29:154–159
Bozzetti F, Mariani L (2009) Defining and classifying cancer cachexia: a proposal by the SCRINIO Working Group. JPEN 33:361–367
Dubrey SW, Cha K, Anderson J et al (1998) The clinical features of immunoglobulin light-chain amyloidosis (AL) with heart involvement. Q J Med 91:141–157
Rutishauser IH (2005) Dietary intake measurements. Public Health Nutr 8:1100–1107
Cereda E, Vanotti A (2008) Short dietary assessment improves muscle dysfunction identification by Geriatric Nutritional Risk Index in uncomplicated institutionalised patients over 70 years old. Clin Nutr 27:126–132
Iversen PO, Wisløff F, Gulbrandsen N (2010) Reduced nutritional status among multiple myeloma patients during treatment with high-dose chemotherapy and autologous stem cell support. Clin Nutr 29:488–491
Arends J, Bodoky G, Bozzetti F et al (2006) ESPEN Guidelines on enteral nutrition: non-surgical oncology. Clin Nutr 25:245–259
Bozzetti F, Arends J, Lundholm K et al (2009) ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr 28:445–454
Conflict of interest
All authors approved the final version of the manuscript and indicated no potential conflicts of interest.
Funding
This study was supported by grants from the Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caccialanza, R., Palladini, G., Klersy, C. et al. Nutritional status independently affects quality of life of patients with systemic immunoglobulin light-chain (AL) amyloidosis. Ann Hematol 91, 399–406 (2012). https://doi.org/10.1007/s00277-011-1309-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1309-x