Abstract
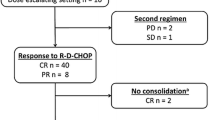
This phase II trial evaluated the efficacy and safety of tandem consolidation using 90yttrium ibritumomab tiuxetan (90Y-IT) and high-dose therapy (HDT) with autologous peripheral blood stem cell transplantation (PBSCT) in high-risk patients with diffuse large B-cell lymphoma (DLBCL) who were in primary remission. Eleven patients with high-risk DLBCL were enrolled. All patients had achieved complete or partial response after six to eight cycles of rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as a frontline chemotherapy. Subsequently, the patients received one to two courses of ifosfamide-containing regimen for peripheral blood stem cell mobilization and harvesting. First consolidation with 90Y-IT was performed, followed by second consolidation using HDT with PBSCT. All patients received 90Y-IT therapy, but three patients did not undergo PBSCT. During the median follow-up period of 18.1 months, 9 of 11 patients exhibited disease progression, and 8 patients died. The estimated 2-year progression-free survival was 18.2%, and overall survival was 36.4%. Adverse events following 90Y-IT consolidation were primarily transient hematologic toxicities. The present pilot study suggests that tandem consolidation therapy using 90Y-IT followed by HDT with autologous PBSCT is not feasible for treatment of high-risk patients with DLBCL in remission after R-CHOP. In addition, this treatment failed to provide beneficial effects for the clinical outcome of subsequent PBSCT.




Similar content being viewed by others
References
Tilly H, Dreyling M (2009) Diffuse large B-cell non-Hodgkin’s lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(4):110–112
Murawski N, Zwick C, Pfreundschuh M (2010) Unresolved issues in diffuse large B-cell lymphomas. Expert Rev Anticancer Ther 10:387–402
Brusamolino E (2009) First-line therapy of CD20+ diffuse large B-cell lymphoma: facts and open questions. Haematologica 94:1194–1198
Fisher RI, Gaynor ER, Dahlberg S et al (1993) Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 328:1002–1006
(1993) A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 329: 987–994
Freedman AS, Takvorian T, Neuberg D et al (1993) Autologous bone marrow transplantation in poor-prognosis intermediate-grade and high-grade B-cell non-Hodgkin’s lymphoma in first remission: a pilot study. J Clin Oncol 11:931–936
Sierra J, Conde E, Montserrat E (1993) Autologous bone marrow transplantation for non-Hodgkin’s lymphoma in first remission. Blood 81:1968–1969
Dillman RO (2002) Radiolabeled anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoma. J Clin Oncol 20:3545–3557
Press WO (2000) Physics for practitioners: the use of radiolabeled monoclonal antibodies in B-cell non-Hodgkin’s lymphoma. Semin Hematol 37:2–8
Harris NL, Jaffe ES, Diebold J et al (1999) World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November 1997. J Clin Oncol 17:3835–3849
Witzig TE, Gordon LI, Cabanillas F et al (2002) Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 20:2453–2463
Tobinai K, Watanabe T, Ogura M et al (2009) Japanese phase II study of 90Y-ibritumomab tiuxetan in patients with relapsed or refractory indolent B-cell lymphoma. Cancer Sci 100:158–164
Morschhauser F, Radford J, Van Hoof A et al (2008) Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol 26:5156–5164
Morschhauser F, Illidge T, Huglo D et al (2007) Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood 110:54–58
Zinzani PL, Tani M, Fanti S et al (2008) A phase II trial of CHOP chemotherapy followed by yttrium 90 ibritumomab tiuxetan (Zevalin) for previously untreated elderly diffuse large B-cell lymphoma patients. Ann Oncol 19:769–773
Zinzani PL, Rossi G, Franceschetti S et al (2010) Phase II trial of short-course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients. Clin Cancer Res 16:3998–4004
Winter JN (2004) Combining yttrium 90-labeled ibritumomab tiuxetan with high-dose chemotherapy and stem cell support in patients with relapsed non-Hodgkin’s lymphoma. Clin Lymphoma 5(Suppl 1):S22–26
Nademanee A, Forman S, Molina A et al (2005) A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood 106:2896–2902
Krishnan A, Nademanee A, Fung HC et al (2008) Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol 26:90–95
Fietz T, Uharek L, Gentilini C et al (2006) Allogeneic hematopoietic cell transplantation following conditioning with 90Y-ibritumomab-tiuxetan. Leuk Lymphoma 47:59–63
Acknowledgments
We are grateful to Bayer Schering Pharma, Seoul, Korea for supplying 90Y-IT (Zevalin®). We would also like to thank Byung-Young Ahn, Geul-Soon Han, and Seung-Won Choi for providing excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eun Ji Han and Sung-Eun Lee equally contributed in this study.
Rights and permissions
About this article
Cite this article
Han, E.J., Lee, SE., Kim, S.H. et al. Clinical outcomes of post-remission therapy using 90yttrium ibritumomab tiuxetan (Zevalin®) for high-risk patients with diffuse large B-cell lymphoma. Ann Hematol 90, 1075–1082 (2011). https://doi.org/10.1007/s00277-011-1191-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1191-6