Abstract
Rapamycin is a potent allosteric mTORC1 inhibitor with clinical applications as an anticancer agent. However, only a fraction of cancer patients responds to the drug, and no biomarkers are available to predict tumor sensitivity. Recently, we and others have obtained evidence for potential involvement of tropomyosin-related kinase (TRK) receptor protein tyrosine kinases (TRKA, TRKB, TRKC) in leukemia. In the present study, we tested the therapeutic effect of Rapamycin and its analog RAD001 on altered TRK-induced leukemia in a murine model. Daily treatment with Rapamycin (2 mg/kg) or RAD001 (1 mg/kg) significantly prolonged the survival of treated animals (n = 40) compared with the placebo group. Consistently, both mTOR and S6 proteins were strongly dephosphorylated in vitro and in vivo after treatment with Rapamycin or RAD001. However, Rapamycin did not completely inhibit mTORC1-dependent phosphorylation of 4E-BP1. With exception of one mouse showing slight reactivation of Akt after treatment, no reactivation of MAPK or Akt pathways was observed in other resistant tumors. Interestingly, leukemic cells isolated from a Rapamycin-resistant mouse were still highly sensitive to Rapamycin in vitro. Our findings suggest that altered TRK signaling may be a good predictor of tumor sensitivity to mTOR inhibition and that pathways other than MAPK and Akt exist that may trigger resistance of leukemic cells to Rapamycin in vivo.





Similar content being viewed by others
References
Tickenbrock L, Muller-Tidow C, Berdel WE, Serve H (2006) Emerging Flt3 kinase inhibitors in the treatment of leukaemia. Expert Opin Emerg Drugs 11:153–165
Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA (2009) Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs 18:1333–1349
Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122:3589–3594
Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA 105:17414–17419
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284:8023–8032
Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, Vu C, Lilly MB, Mallya S, Ong ST, Konopleva M, Martin MB, Ren P, Liu Y, Rommel C, Fruman DA (2010) Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med 16:205–213
Zeng Z, dos Sarbassov D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, Giles FJ, Sabatini DM, Andreeff M, Konopleva M (2007) Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 109:3509–3512
Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, Benzaquen D, Laurent G, Huguet F, Payrastre B (2005) Antileukemic activity of rapamycin in acute myeloid leukemia. Blood 105:2527–2534
Descamps S, Pawlowski V, Revillion F, Hornez L, Hebbar M, Boilly B, Hondermarck H, Peyrat JP (2001) Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res 61:4337–4340
McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD (1999) Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA 96:4540–4545
Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM (1993) Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 328:847–854
Li Z, Dullmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J, Forster M, Stocking C, Wahlers A, Frank O, Ostertag W, Kuhlcke K, Eckert HG, Fehse B, Baum C (2002) Murine leukemia induced by retroviral gene marking. Science 296:497
Reuther GW, Lambert QT, Caligiuri MA, Der CJ (2000) Identification and characterization of an activating TrkA deletion mutation in acute myeloid leukemia. Mol Cell Biol 20:8655–8666
Meyer J, Rhein M, Schiedlmeier B, Kustikova O, Rudolph C, Kamino K, Neumann T, Yang M, Wahlers A, Fehse B, Reuther GW, Schlegelberger B, Ganser A, Baum C, Li Z (2007) Remarkable leukemogenic potency and quality of a constitutively active neurotrophin receptor, deltaTrkA. Leukemia 21:2171–2180
Liu Q, Schwaller J, Kutok J, Cain D, Aster JC, Williams IR, Gilliland DG (2000) Signal transduction and transforming properties of the TEL-TRKC fusions associated with t(12;15)(p13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. Embo J 19:1827–1838
Newrzela S, Cornils K, Li Z, Baum C, Brugman MH, Hartmann M, Meyer J, Hartmann S, Hansmann ML, Fehse B, von Laer D (2008) Resistance of mature T cells to oncogene transformation. Blood 112:2278–2286
Li Z, Beutel G, Rhein M, Meyer J, Koenecke C, Neumann T, Yang M, Krauter J, von Neuhoff N, Heuser M, Diedrich H, Gohring G, Wilkens L, Schlegelberger B, Ganser A, Baum C (2009) High-affinity neurotrophin receptors and ligands promote leukemogenesis. Blood 113:2028–2037
Krutzik PO, Nolan GP (2003) Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A 55:61–70
Wu Q, Kiguchi K, Kawamoto T, Ajiki T, Traag J, Carbajal S, Ruffino L, Thames H, Wistuba I, Thomas M, Vasquez KM, DiGiovanni J (2007) Therapeutic effect of rapamycin on gallbladder cancer in a transgenic mouse model. Cancer Res 67:3794–3800
Morse HC 3rd, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Jaffe ES, Kogan SC, MacLennan IC, Pattengale PK, Ward JM (2002) Bethesda proposals for classification of lymphoid neoplasms in mice. Blood 100:246–258
Xu Q, Thompson JE, Carroll M (2005) mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood 106:4261–4268
Guba M, Koehl GE, Neppl E, Doenecke A, Steinbauer M, Schlitt HJ, Jauch KW, Geissler EK (2005) Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int 18:89–94
Knott PD, Tamai H, Strome M, Van Lente F, Shu S (2007) RAD inhibition of sarcoma growth: implications for laryngeal transplantation. Am J Otolaryngol 28:375–378
Nishioka C, Ikezoe T, Yang J, Gery S, Koeffler HP, Yokoyama A (2009) Inhibition of mammalian target of rapamycin signaling potentiates the effects of all-trans retinoic acid to induce growth arrest and differentiation of human acute myelogenous leukemia cells. Int J Cancer 125:1710–1720
Matsuda C, Ito T, Song J, Mizushima T, Tamagawa H, Kai Y, Hamanaka Y, Inoue M, Nishida T, Matsuda H, Sawa Y (2007) Therapeutic effect of a new immunosuppressive agent, everolimus, on interleukin-10 gene-deficient mice with colitis. Clin Exp Immunol 148:348–359
Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ (2006) Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441:475–482
Crazzolara R, Cisterne A, Thien M, Hewson J, Baraz R, Bradstock KF, Bendall LJ (2009) Potentiating effects of RAD001 (Everolimus) on vincristine therapy in childhood acute lymphoblastic leukemia. Blood 113:3297–3306
Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK, Houghton PJ, Brown VI, Grupp SA (2006) The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood 107:1149–1155
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118:3065–3074
Xing D, Orsulic S (2005) A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. Proc Natl Acad Sci USA 102:6936–6941
Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL (2005) A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood 105:4429–4436
Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL (1995) Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377:441–446
Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B, Baselga J, Ramon y Cajal S (2007) 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res 67:7551–7555
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22:159–168
Acknowledgements
This study was supported by the Deutsche Krebshilfe (grants: 10-2090-Li I and 108245) and DFG excellence cluster REBIRTH. AS is a student of the PhD program for Molecular Medicine in Hannover Medical School. We are very grateful to Rena-Mareike Struß, Jessica Wenzl, and Thomas Neumann for technical assistance; Michael Morgan for critical reading of this paper; and Hans Grundtke, Jörg Frühauf, and Martin Werner (Radiotherapy, Hannover Medical School) for irradiation of animals.
Conflict of interest
Authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mathias Rhein and Adrian Schwarzer contributed equally to this study.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Fig. S1
Phenotyping of #483 cells by FACS analysis. Panels show representative stainings of bone marrow cells (upper row) and thymus cells against markers of lymphoid (CD4, CD8, and CD19) and myeloid lineages (CD11b, Gr1). Additionally, thymus cells were further characterized, showing expression of CD25, CD44 and CD3 (all lymphoid lineage), but not Ter119 (data not shown). The #483 cell line was established from thymus cells of a mouse transplanted with a retroviral vector expressing ∆TrkA and eGFP [1] (PDF 248 kb)
Fig. S2
Establishment of a murine model for in vivo application of Rapamycin. a Kaplan–Meier plot for the titration of #483 cells. After sublethal irradiation with 7.5 Gy, 4 C57Bl/6J mice per group were transplanted with 107 (dashed line, mean survival 21 days), 106 (dotted line, mean survival 35 days), or 105 (solid line, survived observation time) cells per mouse. All animals with 107 cells died on day 21 after transplantation, and 50% of the animals from the 106 group died around day 35, while none of the 105 group developed leukemia after observation for 8 months. Thus, we chose to transplant animals with 106 cells in further experiments. b Rapamycin levels in whole blood. Blood samples of daily treated healthy mice (2 mg/kg Rapamycin) via intraperitoneal injection (i.p.) were taken at the respective time points. Rapamycin levels are comparable with levels achieved in other murine models (15–72 ng/ml) [2] and patients (70 ng/ml max). c Leukocytes (WBC) in peripheral blood of animals treated with Rapamycin. d Pharmacokinetic study of RAD001 in mice: steady state RAD001 levels after i.p. injection of three different doses. e Treatment of RAD001 induced slight leukopenia of healthy mice treated with RAD001. (PDF 1324 kb)
Fig. S3
Kaplan–Meier plot of animals treated with Rapamycin. Animals were transplanted with #481 cells [1]. The progression of leukemia was significantly delayed in the cohort treated with apamycin (p < 0.05) (PDF 162 kb)
Fig. S4
Monitoring of leukemia cells in peripheral blood of animals. In placebo group (a), the population of GFP positive cells went up in two animals 1 week after treatment with carrier (the rest of the group died around 1 week after treatment start). In RAD001 treated animals (b), the leukemic population went down initially, but eventually rose up (PDF 133 kb)
Fig. S5
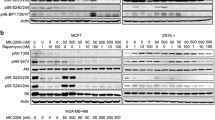
a Western blots of Rapamycin-treated cells. #483 cells were incubated with inhibitor for 24 h and lysed using standard procedures. There is a strong reduction of phosphorylated protein in #483 cells for S6 protein, a downstream substrate of mTOR. There was no reactivation of ERK. b PhosFow analysis showed no reactivation of Akt or ERK in #483 cells treated with Rapamycin in vitro. c Reduced phosphorylation of 4E-BP1 (Thr-37/46) in #483 cells after treatment. However, phosphorylation of Ser-65 was not significantly changed. Gel shifts can be observed in samples treated with Rapamycin. The α-β-γ isoforms represent the phosphorylation status of 4E-BP1 with α being hypophosphorylated and γ being hyperphosphorylated [3]. d No reactivation of Akt in Rapamycin-treated animal (#1011) in comparison with a control animal (#1009). e Slightly high activation of Akt in mouse #958, but not in #840 (both Rapamycin-treated). #953 is from the placebo group. Note the higher activation of the parent cells #483 in vitro (PDF 560 kb)
ESM 1
(DOC 24 kb)
Rights and permissions
About this article
Cite this article
Rhein, M., Schwarzer, A., Yang, M. et al. Leukemias induced by altered TRK-signaling are sensitive to mTOR inhibitors in preclinical models. Ann Hematol 90, 283–292 (2011). https://doi.org/10.1007/s00277-010-1065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-010-1065-3