Abstract
Umbilical cord blood transplantation (UCBT) with two units has been conducted with promising results in adults to overcome the limitation of low cell numbers. In an attempt to improve the outcomes, double UCBT was performed in children and adolescents. Sixty-one patients, including 44 acute leukemia, and 17 other hematologic diseases, received double UCBT. Donor-type engraftment achieved in 82% of patients. Except one patient with persistent mixed chimerism of two units, other 49 patients showed dominancy of one unit and only the CFU-GM was significant factor influencing dominancy. The event-free survival (EFS) of leukemia and other hematologic disease were 59% and 53%, respectively, and the EFS of acute leukemia patients who received transplant in first or second CR (68.6%) was significantly better than in those with advanced disease (22.2%) (P = 0.007). Among the factors influencing outcomes, low cell dose difference between two units (TNC difference/TNC of large unit <15%) were associated with higher TRM, relapse, and lower EFS. Double UCBT was a promising modality of transplant in children and adolescence. However, engraftment and other results were not so satisfactory yet. To improve the outcomes, development of new selection guideline, probably including cell dose difference between two units and technology to enhance engraftment and reduce transplantation-related mortality are warranted.
Similar content being viewed by others
Introduction
Since the first successful transplantation of umbilical cord blood cells was used to treat a patient with Fanconi anemia in 1988 [1], umbilical cord blood transplantation (UCBT) has become an alternative to bone marrow transplantation (BMT) in the treatment of a variety of diseases. Recent series of studies revealed the promising outcomes of cord blood transplantation that are comparable to the results for transplant of unrelated bone marrow (BM) [2–6] .
Cord blood cells have many theoretical advantages as grafts for stem cell transplantation because of their immaturity. Compared with those of adults, umbilical cord blood (UCB) stem cells produce larger in vitro hematopoietic colonies, and can be expanded in long-term culture in vitro. The properties of UCB cells should theoretically compensate for the relatively low numbers of cells contained in a single UCB unit, and through rapid expansion reconstitute myeloablated patients with 1–2 logs fewer nucleated cells than bone marrow. However, low cell numbers compromise outcome if infused cell doses fall below critical limits [7]. To enhance engraftment, ex vivo expansion of UCB cells has been attempted in several studies with limited success [8]. Recently, the transplantation of two partially HLA-matched UCB units has been attempted in adults with promising engraftment result [9], but this method is at an early stage of development and little is known about the underlying mechanism of double UCBT.
Engraftment failure is a problem when using a single unit even in pediatric patients with relatively lower body weight than adults as shown in a recent prospective study of COBLT [10]. In an attempt to improve outcomes, we performed UCBT with two units in pediatric patients and present the analysis of the results and the factors that affect the outcomes.
Patients and methods
Patients and cord blood unit selection
A total of 61 patients diagnosed with acute leukemia [25 acute myeloid leukemia (AML), 19 acute lymphoblastic leukemia (ALL)] including nine advanced disease [third complete remission (CR), refractory disease, second transplantation] or other hematologic diseases were given transplants of two UCB units consecutively at Seoul National University Children's Hospital and Samsung Medical Center between March 2004 and August 2007. Matched related or unrelated BM or peripheral blood stem cell donor was not available in all patients. Median age and body weight of patients (43 male and 18 female) were 9 years (1–18 years) and 32 kg (9–72 kg), respectively. The clinical characteristics of patients are summarized in Table 1. Cord blood units were selected based on molecular typing of 2 digits for human leukocyte antigen (HLA)-A, -B, and -DRB1 loci, and total nucleated cells (TNC) dose. Patients received two units of cord blood that were matched with the recipient and with each other for 4–6/6 HLA-A, -B, or -DRB1 loci and TNC with summing numbers in paired units more than 3.0 × 107/kg at freezing. In the early period of study, patients without appropriate single cord blood unit (TNC<2.0×107/kg) were received double UCBT, but after promising result of preliminary result [11], all UCBT was done with two units. Written informed consent was obtained from all patients prior to transplantation. The use of human material for scientific purposes in this study was approved by the Institutional Review Board of Seoul National University Hospital (0603-142-170).
Treatment
Patients received various conditioning regimens and graft-versus-host disease (GVHD) prophylaxis according to their diseases status and supportive care accorded with the guidelines for stem cell transplantation at each center (Table 1) [11, 12]. All patietns received myeloablative dose of total body irrdiation or busulfan except four patients who received total body (or lymphoid) irradiation (4 Gy), fludarabine (180 mg/m2) and intravenous (i.v.) busulfan (6.4 mg/kg) including three acute leukemia and one aplastic anemia [11].
Analysis of granulocyte-macrophage colony-forming units
For assay of granulocyte-macrophage colony-forming units (CFU-GM), the cell suspension was centrifuged at 1,500 r.p.m. for 10 min, washed once, then a cell count was performed and 1 ml of a suspension of 5 × 105 nucleated cells prepared, of which 0.2 ml (1 × 105 nucleated cells) was mixed with 2 ml of a progenitor assay medium or 0.3 ml (1.5 × 105 nucleated cells) was mixed with 3 ml of a progenitor assay medium (MethoCult GF H4544, StemCell Technologies, Vancouver, Canada). Finally, the samples containing 1 × 105 or 0.5 × 105 nucleated cells were plated in triplicate in 35 × 10 mm culture dishes. The dishes were incubated at 37°C in a humidified 5.5% CO2 incubator for 14 days and colonies were counted using an inverted microscope.
Engraftment and chimerism analysis
Myeloid engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) of 0.5 × 109/l, and platelet recovery was defined to have occurred when the platelet count reached 20 × 109/l without transfusion. Hematopoietic chimerism of BM was evaluated by serial analysis of short tandem repeats at 1, 3, 6 months, and 1 year after UCBT. The analytic method used was based on the quantitative amplification of informative polymorphic short tandem repeat regions in the recipient and donor using AmpFlSTR profiler PCR amplification kit (Applied Biosystems, Foster City, CA) as per the manufacturer's instructions. Engraftment required hematologic recovery from donor-origin cells and the dominant winner unit was determined by chimerism analyses as the predominating donor unit.
Statistics
To compare the differences between means of cell doses and GFU-GM in dominant and non-dominant units, a Wilcoxon's signed-rank test was used. Differences between categorical variables were analyzed by Chi-square test. The Kaplan–Meier method and log-rank univariate comparisons were used to calculate the incidence of GVHD and engraftment and to evaluate overall survival, event-free survival (EFS), relapse rate, and transplantation-related mortality (TRM) rate. Transplantation-related mortality, leukemia relapse, and primary engraftment failure without autologous recovery were considered as events. For multivariate analysis of prognostic factors affecting survival, a Cox proportional hazard regression model was used. Events were defined as engraftment failure, disease progression, relapse or TRM. SPSS version 17.0 was used for all statistical analyses, and statistical significance was accepted when P < 0.05.
Results
Engraftment
The median number of infused TNC, CD34+ cells, CD3+ cells, and CFU-GM, which were obtained by summing numbers in paired units after thawing, were 5.4 × 107/kg (0.7–18.4 × 107/kg), 2.1 × 105/kg (0.6–7.2 × 105/kg), 0.7 × 107/kg (0.1–3.4 × 107/kg), and 0.7 × 105/kg (0.0–27.0 × 105/kg), respectively. Donor-type engraftment (>90%) achieved in 50/61 patients (82.0%) excluding five engraftment failures (failure of donor-type engraftment without hematologic recovery), five autologous recoveries (failure of donor-type engraftment with recipient type hematologic recovery), and one early death. All autologous recovery occurred in other hematologic disease (two juvenile myelomonocytic leukemia, osteopetrosis, adrenoleukodystrophy, and congenital dyserythropoietic anemia). The median number of days required to reach an ANC of more than 0.5 × 109/l or 1.0 × 109/l were 18 days (12–37 days) and 20 days (12–46 days), respectively. Spontaneous platelet recovery to more than 20 × 109/l or 50 × 109/l required a median 46 days (26–137 days) and 57 days (27–306 days), respectively, excluding seven patients (who achieved WBC engraftment but died before recovery of platelet). Sum of TNC dose (less or more than median) did not affect the myeloid engraftment (P = 0.90). Although the number of pre-thawing TNC was significantly correlated with post-thawing TNC (R = 0.91, P = 0.00), nine patients received sum of post-thawing TNC less than planned dose (3.0 × 107/kg), and one of them with sum of TNC 2.7 × 107/kg failed in engraftment. The myeloid engraftment of patients with acute leukemia (90.1%) was better than those with other hematologic diseases (62.5%) (P = 0.03). But the platelet engraftment in patients with acute leukemia (85.3%) was not significantly different from those with hematologic diseases (65.3%) (P = 0.246).
Determination of dominancy and associated factors
All of the 50 patients who achieved donor-type engraftment showed dominance of one of the two units, except for one patient who showed persistent mixed chimerism of two cord blood units. We analyzed the factors associated with the determination of dominance in 44 patients who had all data of number of infused TNC, CD34+ cells, CD3+ cells and CFU-GM, and only the CFU-GM was significant factor influencing dominancy (Table 2).
Graft-versus-host disease
Grades II–IV acute GVHD occurred in 52% of patients, with the majority being grade II and grades III–IV acute GVHD occurring in only 10% of the 50 patients who achieved donor-type engraftment. Of the 44 patients who survived 100 days after UCBT, chronic GVHD occurred in 19 (43.2%) patients, including limited disease in nine (20.5%) and extensive disease in ten (22.7%).
Survival data
The EFS of the 61 patients was 57.4%. The EFS for acute leukemia and other hematologic diseases were 59.1% and 52.9%, respectively. Among acute leukemia patients the EFS was significantly better in patients who received transplant in first or second CR (68.6%) than in those with advanced disease (22.2%) (P = 0.007). In ALL, the EFS was significantly worse in patients with advanced disease (0%) than in those with first CR (66.7%) or second CR (66.7%) (P = 0.007). However, there was no difference in EFS in AML patients with advanced disease (40.0%), first CR (75.0%) or second CR (50.0%) (P = 0.447) (Fig. 1).
Survival data. a Event-free survival (EFS) of 61 patients was 57.4%. b The EFS of acute leukemia and other hematologic disease was 59.1% and 52.9%, respectively. (P = 0.524). c Among acute leukemia patients the EFS was significantly better in patients who received UCBT in first or second complete remission (CR) (68.6%) than in those with advanced disease (22.2%) (P = 0.007). d The EFS was significantly worse in ALL patients with advanced disease (0%) than those in first CR (66.7%) or second CR (66.7%) (P = 0.007). e In AML patients, there was no difference in EFS in patients with advanced disease (40.0%), first CR (75.0%) or second CR (50.0%) (P = 0.447)
Relapse rate
The relapse rate for the 44 acute leukemia patients was 19.0%. The relapse rate of patients with advanced disease was significantly higher (37.8%) than of patients in first or second CR (13.8%). In ALL, patients with advanced disease (50%) showed significantly higher relapse rate than those in CR1 (14.3%) or CR2 (0%) (P = 0.014). There was no difference between AML patients with advanced disease (25.0%), first CR (13.4%) or second CR (33.4%) (P = 0.763).
Infection and transplantation-related mortality
Cytomegalovirus (CMV) infection and CMV disease developed in 34 (55.7%) and 15 (24.6%) patients, respectively. CMV diseases were pneumonia in six patients, retinitis in five, enteritis in three, and hepatitis in one patient. Two patients developed posttransplantation lymphoproliferative disorder (PTLD). Fifteen patients died of TRM and the causes of TRM are illustrated in Table 3.
HLA match and unit selection
The guidelines for selection of cord blood units in this study is somewhat different from that of the Minnesota group, which selected the first UCB unit to be matched with the recipient for 4–6/6 HLA-A, -B, and -DRB1, and the second unit had to be matched to both the recipient and the first unit for 4–6/6 HLA-A, -B, and -DRB1 using intermediate-resolution antigen level typing for A and B and high-resolution for DR [9, 13].
In our guidelines, we selected the UCB units using two-digit HLA-DR typing. High-resolution DR typing was performed in 56 patients, of which only 37 (66%) patients' typing was consistent with the selection guidelines of the Minnesota group. However, the survival of patients who received UCB units selected in accordance with the Minnesota guidelines (56.8%) and that of more mismatched patients (63.2%) was not significantly different (P = 0.527). In 39 acute leukemia patients, the degree of HLA matching in accordance with (58.3%) or different from (66.7%) Minnesota guidelines also did not affect the EFS (P = 0.596). The incidence of chronic GVHD in patients who received UCB units selected in accordance with the Minnesota guidelines (10/25) and that of more mismatched patients (6/10) was not significantly different (P = 0.873).
Factors associated with outcomes
Engraftment was an important factor for EFS. In 61 patients, the patients who failed to engraft (27.3%) showed significantly lower survival than engrafted patients (64.0%) (P = 0.000). In acute leukemia, 4 patients who failed to engraft (0%) also had significantly worse EFS than those who did engraft (65.0%) (P = 0.000) and all failed patients died although two of them received salvage transplantation. As mentioned previously, sum of TNC dose (less or more than median) did not affect the engraftment and the EFS (56.7%) of patients received UCB units with lower sum of TNC (less than median) was not different from that of others (58.1%) (P = 0.953). The EFS of 9 patients received UCB units with sum of post-thawing TNC less than 3 × 107/kg (44.4%) was not different from that of others (59.6%) (P = 0.342).
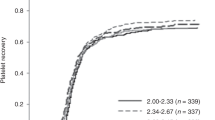
We analyzed the cell dose difference between the two units and found that a low cell dose difference (TNC difference/TNC of large unit <15%) was a significant prognostic factor. The EFS of patients who received two UCB units with a low cell dose difference (25.0%) was significantly worse than that of others (73.2%) (P = 0.000). The TRM of patients with a low cell dose difference (56.5%) was higher than that of other patients (13.2%), significantly (P = 0.001). The relapse rate of acute leukemia was also significantly higher in patients with a low cell dose difference (50.6%) than in others (7.7%) (P = 0.002) (Fig. 2).
The low cell dose difference (TNC difference/large unit <15%) between two units and outcomes. a The EFS of patients who received two UCB units with a low cell dose difference (25.0%) was significantly worse than that of other patients (73.2%) (P = 0.000). b The TRM of patients with a low cell dose difference (56.5%) was higher than that of other patients (13.2%), significantly (P = 0.001). c The relapse rate of acute leukemia was significantly higher in patients with a low cell dose difference (50.6%) than in others (7.7%) (P = 0.002)
Discussion
In present study, we performed double UCBT in pediatric patients including AML in first CR according to the guideline of Korean National Health Insurance Review & Assessment which allow UCBT for patients without appropriate donor in first CR. Although there is some controversy about the role of alternative donor transplantation including UCBT for AML in first CR [14–17], pediatric AML patients without matched related donor routinely undergo alternative donor transplantation in first CR in Korea as the outcome of chemotherapy was not satisfactory [18], the allogeneic transplantation is associated with improved outcomes in first CR [17], and promising results of alternative donor transplantation were demonstrated in pediatric AML with the development of transplantation techniques [16, 19].
We choose double UCBT for patients without an appropriate related or unrelated donor based on the comparable results of unrelated UCBT and BMT [3, 17]. Double UCBT is a promising method because this kind of transplant has been proposed to have better engraftment potential than single UCBT and thus offer a better chance of survival, although the exact mechanism is not yet known [9, 11, 13, 20].
Our previous result of one center showed the dominancy of one unit in all transplant in early period of engraftment, which concurs with a previous report by the Minnesota group [9, 11]. However, the first patient in the present study (and in Korea) has survived for more than 5 years, and showed mixed chimerism (about 70:30) of both units for 2 years after which one unit disappeared. It has also been reported that mixed chimerism was maintained for as long as 479 days after a double UCBT before dominant reversion was achieved, although the exact mechanism is not clear [21]. In our present extended study, persistent mixed chimerism of both units (about 60:40) was detected in one patient for more than 3 years, the longest mixed chimerism described in the literature. However, all other patients with donor-type engraftment showed dominance of one of the two units.
It is not known why one unit becomes the winner. Barker et al. reported that a larger CD3+ cells dose was associated with dominance, but with some exceptions [9]. It is possible that the unit with the larger number of CD3+ cells might defeat the “weaker” unit, but in our study CD3+ cells dose did not affect the determination of the winner. This was also concordant with the later data of the Minnesota group in an investigation of a larger cohort of patients [13]. In our previous report, comparison of the infused CFU-GM number of the winner unit versus that of the loser unit in 18 double UCBT revealed that the CFU-GM dose of the winner unit was significantly greater [12]. Although the methods of measuring the CFU-GM were somewhat different between the two centers and the median value skewed to low side as the mean CFU-GM of one center is significantly lower than others, in the present study with a larger number of patients, it was the sole factor affecting the determination of dominance. We could not, however, determine the prognostic significance of the CFU-GM.
In a recent report from the Minnesota group, transplant of two UCB units was shown to be a risk factor for acute GVHD. The cumulative grade II–IV acute GVHD of double UCBT (58%) was significantly higher than that of single UCBT (39%), although the incidence of more severe grades III–IV acute GVHD was similar between the groups (19% with double unit and 18% with single unit UCB grafts) [22]. In our study, grades II–IV acute GVHD occurred in 52% of patients, but the majority of this was grade II; grades III–IV acute GVHD occurred in only 10% of patients. These data were similar to that of the Minnesota group. Although the precise mechanism is not known, the Minnesota group suggests that the increased incidence of acute GVHD after double UCBT may be caused by a graft-versus-graft effect, similar to an in vivo mixed lymphocyte reaction.
The high incidence of grade II acute GVHD in double UCBT might be associated with the graft-versus-leukemia effect. Recently, the Eurocord–Netcord/EBMT and Minnesota groups reported results demonstrating the potential impact of double UCBT on the risk of relapse [23, 24]. The Minnesota group analyzed the outcome of UCBT for acute leukemia and showed a significantly lower relapse rate for double UCBT (19%) than single UCBT (33%) [24]. In our study the relapse rate of 44 acute leukemia patients was also 19.0%. The relapse rate of patients in first or second CR was 13.8%, which is also similar to that (16%) of the Minnesota group. This group speculates that increased alloreactivity, the mechanism and effector cells of which remain unclear, may be induced by the graft–graft interaction between the two UCB units, and that this may be responsible for the reduced risk of relapse.
Eapen et al reported 18% of chronic GVHD in a large pediatric study of single unit UCBT and the incidences of chronic GVHD of full matched (10/33), one-antigen mismatched (34/186), and two-antigen mismatched (38/247) UCBT were not so different in that report [3]. The incidence of chronic GVHD in our pediatric study (43.2%) was rather higher than that of report by Eapen et al [3], and also higher than the incidence (18%) of adult double UCBT study by Minnesota group [24], but we could not find associated factor for chronic GVHD including HLA matching in our study. Effort to find the risk factor for chronic GVHD is needed with larger number of pediatric patients.
CMV reactivation is one of the major concerns of UCBT, occurring in more than half the patients receiving UCBT [25–27], although some studies have reported that recipients of UCBT have a similar risk of CMV infection as do recipients of other types of transplant [28, 29]. In the present study, CMV infection was the number one cause of TRM and was documented in 34 (56%) patients, five of whom died (four pneumonia, one hepatitis). Because ganciclovir is an agent presented in an inconvenient intravenous form and has myelosuppressive activity, raising the possibility of neutropenia and bacterial infection [30], the oral formula of acyclovir with equivalent preventive effect for CMV was used in this study [31]. In our previous analysis, CMV antigenemia was a risk factor for pulmonary complications and acute respiratory distress syndrome in hematopoietic stem cell transplantation, and we recommended aggressive prophylaxis and treatment of CMV infection [32]. Prophylaxis with oral valganciclovir was proposed to be as safe and effective as intravenous ganciclovir for preventing CMV infection after UCBT, but valganciclovir also has myelosuppressive activity and a significantly higher number of patients required granulocyte colony stimulating factor after engraftment than those treated with acyclovir [33]. Recently, Hanley et al. reported successful in vitro expansion of CMV antigen-specific T cells from UCB T cells with a naive phenotype [34]. Introduction of this kind of functionally active virus-specific T cells will improve the outcomes of UCBT.
We experienced PTLD in two patients, including TRM in one patient who did not receive antithymocyte globulin (ATG). A recent analysis found no significant difference in the risk of serious viral infections, including PTLD, in recipients of unrelated UCB or unmanipulated marrow [35]. However, the incidence of EBV-related complications was reported to be significantly higher in a subset of patients treated with a nonmyeloablative preparative regimen that included ATG, although overall survival was not different [36]. ATG is a known risk factor for EBV infection [37], and more than 75% (46/61) of the patients in our study received ATG, but as we did not perform regular EBV monitoring in the early period of this study, we could not analyze the incidence of EBV. Recently, Heslop proposed that early intervention or even prophylactic administration may be warranted where there is a high risk of developing PTLD, such as a patient who has received potent T cell depleting or suppressing antibodies [38]. We have now introduced preemptive therapy with single dose rituximab for patients who receive ATG showing high EBV DNA levels to prevent EBV-associated PTLD [39, 40].
The most interesting result from our data on double UCBT was the prognostic significance of cell dose difference between the two units. We found that a lower cell dose difference (TNC difference/TNC of large unit <15%) significantly affected TRM, relapse, and EFS. In the setting of double UCBT, mixed lymphocyte reaction might occur between the two fresh but immature UCB units. If the strength of the two UCB units is similar, they will be exhausted after the fight and possibly lose their power to fight the leukemia and pathogens. Although this is only a hypothesis that needs to be validated scientifically and clinically, analysis of the cell dose difference between two units may be easy for other researchers who perform double UCBT.
In some patients, the engrafted unit had a lower cell dose than the loser unit, but the exact mechanism of this is unknown. In future, analysis of subsets of hematopoietic cells and immune cells in UCB units including T cell subsets, regulatory T cells, NK cells, and NK/T cells may give an insight into this complex type of transplantation.
In relation to current recommendations for UCB unit selection, Gluckman commented that if there is no single appropriate unit available, it is suggested that one should look for two units with a combined total dose of ≥3 × 107 nucleated cells/kg [41]. In our study, nine of 61 patients received two UCB units with sum of post-thawing TNC less 3 × 107/kg although we selected cord blood units with sum of TNC more than 3 × 107/kg at freezing. But sum of TNC did not affect EFS and this result needs to be verified again with large number of patients received double UCBT in future.
The Minnesota group selected a first UCB unit that was matched to the recipient for 4–6/6 HLA-A, -B, and -DRB1 and a second unit that had to be matched to both the recipient and the first unit for 4–6/6 HLA-A, -B, and -DRB1 using intermediate-resolution antigen level typing for HLA-A and -B and high-resolution for DR [9, 13].
HLA matching is very important in single unit UCBT and with the HLA-mismatched antigen increased, the rate of graft failure, severe GVHD and TRM increased, and the DFS decreased [42].
Although HLA matching with high-resolution typing has been emphasized in bone marrow and mobilized peripheral blood transplantation, high-resolution typing for HLA-A and -B did not improve long-term clinical outcomes after UCBT [43, 44]. In HLA-DR typing, there is some controversy about the importance of high-resolution typing for UCBT [45]. In this study, we selected UCB units using two-digit HLA-DR typing. When we reanalyzed the results according to the HLA mismatch with high-resolution DR typing, the survival of patients who received UCB units selected in accordance with the Minnesota guidelines was not different from more mismatched patients. Except in our study, selection of the two units in other double UCBT studies are based on the Minnesota guidelines with high-resolution DR typing. Double UCBT has different immune and engraftment mechanisms from single UCBT and the role of high-resolution DR typing in the setting of two units transplantation should be clarified in the future.
Multiple factors are associated with the outcomes of UCBT, including those factors that affect the recipient environment (underlying disease, previous treatment, conditioning regimen, use of ATG, and the method of GVHD prophylaxis), the characteristics of each UCB unit, and the HLA disparity between graft and host. For this reason, the selection of the two units requires consideration of all the above factors. However, optimal selection guidelines that take into account the influence of these factors are not available. In this study, the selection of units with similar cell doses resulted in poor outcomes. Selection of one strong unit with high cell numbers and one weak unit to activate the strong one, and thus create dominance of one unit, might result in a better outcome than the combination of two strong units.
In conclusion, double UCBT is a promising modality of transplant in children and adolescents. However, engraftment and other results are not yet satisfactory. To improve the outcomes, the development of new selection guidelines, probably including cell dose difference between the two units, and the development of technology to enhance engraftment and reduce transplantation-related mortality are warranted.
References
Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P et al (1989) Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 321:1174–1178
Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S, Tiedemann K, Busca A, Chan KW, Kato S, Ortega J, Vowels M, Zander A, Souillet G, Oakill A, Woolfrey A, Pay AL, Green A, Garnier F, Ionescu I, Wernet P, Sirchia G, Rubinstein P, Chevret S, Gluckman E (2001) Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood 97:2962–2971
Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM, Wagner JE (2007) Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 369:1947–1954
Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M, Finke J, Frassoni F, Gluckman E (2004) Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 351:2276–2285
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, van Rood JJ, Scaradavou A, Horowitz MM (2004) Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 351:2265–2275
Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, Fukuno K, Uchiyama M, Takasugi K, Iseki T, Tojo A, Yamaguchi T, Asano S (2007) Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood 109:1322–1330
Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM (2002) Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100:1611–1618
Barker JN, Wagner JE (2003) Umbilical-cord blood transplantation for the treatment of cancer. Nat Rev Cancer 3:526–532
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, Verfaillie CM, Wagner JE (2005) Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 105:1343–1347
Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lowe LA, Wall D, Kapoor N, Guinan EC, Feig SA, Wagner EL, Kernan NA (2008) Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood 112:4318–4327
Kang HJ, Kho SH, Jang MK, Lee SH, Shin HY, Ahn HS (2006) Early engraftment kinetics of two units cord blood transplantation. Bone Marrow Transplant 38:197–201
Yoo KH, Lee SH, Kim HJ, Sung KW, Jung HL, Cho EJ, Park HK, Kim HA, Koo HH (2007) The impact of post-thaw colony-forming units-granulocyte/macrophage on engraftment following unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplant 39:515–521
Majhail NS, Brunstein CG, Wagner JE (2006) Double umbilical cord blood transplantation. Curr Opin Immunol 18:571–575
Michel G, Rocha V, Chevret S, Arcese W, Chan KW, Filipovich A, Takahashi TA, Vowels M, Ortega J, Bordigoni P, Shaw PJ, Yaniv I, Machado A, Pimentel P, Fagioli F, Verdeguer A, Jouet JP, Diez B, Ferreira E, Pasquini R, Rosenthal J, Sievers E, Messina C, Iori AP, Garnier F, Ionescu I, Locatelli FGluckman E (2003) Unrelated cord blood transplantation for childhood acute myeloid leukemia: a Eurocord group analysis. Blood 102:4290–4297
Oliansky DM, Rizzo JD, Aplan PD, Arceci RJ, Leone L, Ravindranath Y, Sanders JE, Smith FO 3rd, Wilmot F, McCarthy PL, Hahn T Jr (2007) The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transplant 13:1–25
Anasetti C, Perkins J, Nieder MLField T (2006) Are matched unrelated donor transplants justified for AML in CR1? Best Pract Res Clin Haematol 19:321–328
Hwang WY, Samuel M, Tan D, Koh LP, Lim WLinn YC (2007) A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant 13:444–453
Lee DH, Chung NG, Cho B, Kim HK, Kang HJ, Shin HY, Ahn HS, Yoo KH, Sung KW, Koo HH, Kook H, Hwang TJ, Im HJ, Seo JJPark HJ (2010) Idarubicin plus behenoyl cytarabine and 6-thioguanine compares favorably with idarubicin plus cytarabine-based regimen for children with previously untreated acute myeloid leukemia: 10-year retrospective, multicenter study in Korea. J Korean Med Sci 25:9–15
Lee DH, Kwon YJ, Lim J, Kim Y, Han K, Chung NG, Jeong DC, Cho BKim HK (2009) Comparable outcomes of HLA-matched unrelated and HLA-identical sibling donor bone marrow transplantation for childhood acute myeloid leukemia in first remission. Pediatr Transplant 13:210–216
Ruggeri A, de Latour RP, Rocha V, Larghero J, Robin M, Rodrigues CA, Traineau R, Ribaud P, Ferry C, Devergie A, Gluckman E, Socie G (2008) Double cord blood transplantation in patients with high risk bone marrow failure syndromes. Br J Haematol 143:404–408
Yen HJ, Chiou TJ, Hung GY, Chang CY, Hsieh MY, Tzeng CH, Chen PM, Tang RB (2008) Long-term mixed full-donor chimerism with dominance reversion after a double-unit cord blood transplant. Eur J Haematol 80:366–367
MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, Blazar BR, Wagner JE (2009) Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood 113:2410–2415
Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, de Lima M, Cairo MS, Furst S, Rio B, Dalley C, Carreras E, Harousseau JL, Mohty M, Taveira D, Dreger P, Sureda A, Gluckman E, Rocha V (2009) Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 27:256–263
Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, Burke MJ, Blazar BR, Miller JS, McGlave PB, Weisdorf DJ, Wagner JE (2009) Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 114:4293–4299
Beck JC, Wagner JE, Defor TE, Brunstein CG, Schleiss MR, Young JA, Weisdorf DH, Cooley S, Miller JS, Verneris MR (2010) Impact of Cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant 16:215–222
Matsumura T, Narimatsu H, Kami M, Yuji K, Kusumi E, Hori A, Murashige N, Tanaka Y, Masuoka K, Wake A, Miyakoshi S, Kanda Y, Taniguchi S (2007) Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biol Blood Marrow Transplant 13:577–583
Takami A, Mochizuki K, Asakura H, Yamazaki H, Okumura H, Nakao S (2005) High incidence of cytomegalovirus reactivation in adult recipients of an unrelated cord blood transplant. Haematologica 90:1290–1292
Walker CM, van Burik JA, De For TE, Weisdorf DJ (2007) Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant 13:1106–1115
Yoon HS, Lee JH, Choi ES, Seo JJ, Moon HN, Kim MN, Im HJ (2009) Cytomegalovirus infection in children who underwent hematopoietic stem cell transplantation at a single center: a retrospective study of the risk factors. Pediatr Transplant 13:898–905
Parody R, Martino R, Rovira M, Vazquez L, Vazquez MJ, de la Camara R, Blazquez C, Fernandez-Aviles F, Carreras E, Salavert M, Jarque I, Martin C, Martinez F, Lopez J, Torres A, Sierra J, Sanz GF (2006) Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant 12:734–748
Burns LJ, Miller W, Kandaswamy C, DeFor TE, MacMillan ML, Van Burik JA, Weisdorf DJ (2002) Randomized clinical trial of ganciclovir vs acyclovir for prevention of cytomegalovirus antigenemia after allogeneic transplantation. Bone Marrow Transplant 30:945–951
Lee JW, Kang HJ, Park JD, Shin HY, Ahn HS (2009) Early pulmonary complications after hematopoietic stem cell transplantation in pediatric patients: association with cytomegalovirus infection. J Pediatr Hematol Oncol 31:545–551
Montesinos P, Sanz J, Cantero S, Lorenzo I, Martin G, Saavedra S, Palau J, Romero M, Montava A, Senent L, Martinez J, Jarque I, Salavert M, Cordoba J, Gomez L, Weiss S, Moscardo F, de la Rubia J, Larrea L, Sanz MA, Sanz GF (2009) Incidence, risk factors, and outcome of cytomegalovirus infection and disease in patients receiving prophylaxis with oral valganciclovir or intravenous ganciclovir after umbilical cord blood transplantation. Biol Blood Marrow Transplant 15:730–740
Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, Decker W, Molldrem JJ, Liu H, Gee AP, Rooney CM, Heslop HE, Dotti G, Brenner MK, Shpall EJ, Bollard CM (2009) Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood 114:1958–1967
Barker JN, Hough RE, van Burik JA, DeFor TE, MacMillan ML, O'Brien MR, Wagner JE (2005) Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant 11:362–370
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, Wagner JE (2006) Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 108:2874–2880
Juvonen E, Aalto S, Tarkkanen J, Volin L, Hedman K, Ruutu T (2007) Retrospective evaluation of serum Epstein Barr virus DNA levels in 406 allogeneic stem cell transplant patients. Haematologica 92:819–825
Heslop HE (2009) How I treat EBV lymphoproliferation. Blood 114:4002–4008
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW, Verdonck LF, Lowenberg B, Cornelissen JJ (2002) Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 99:4364–4369
Gruhn B, Meerbach A, Hafer R, Zell R, Wutzler P, Zintl F (2003) Pre-emptive therapy with rituximab for prevention of Epstein-Barr virus-associated lymphoproliferative disease after hematopoietic stem cell transplantation. Bone Marrow Transplant 31:1023–1025
Gluckman E, Rocha V (2009) Cord blood transplantation: state of the art. Haematologica 94:451–454
Shi-xia X, Xian-hua T, Hai-qin X, Bo F, Hai-qing C, Xiang-Feng T (2009) Meta-analysis of HLA matching and the outcome of unrelated umbilical cord blood transplantation (CBT). Transpl Immunol 21:234–239
Kogler G, Enczmann J, Rocha V, Gluckman E, Wernet P (2005) High-resolution HLA typing by sequencing for HLA-A, -B, -C, -DR, -DQ in 122 unrelated cord blood/patient pair transplants hardly improves long-term clinical outcome. Bone Marrow Transplant 36:1033–1041
Kamani N, Spellman S, Hurley CK, Barker JN, Smith FO, Oudshoorn M, Bray R, Smith A, Williams TM, Logan B, Eapen M, Anasetti C, Setterholm M, Confer DL (2008) State of the art review: HLA matching and outcome of unrelated donor umbilical cord blood transplants. Biol Blood Marrow Transplant 14:1–6
Liao C, Wu JY, Xu ZP, Li Y, Yang X, Chen JS, Tang XW, Gu SL, Huang YN, Tang PH, Tsang KS (2007) Indiscernible benefit of high-resolution HLA typing in improving long-term clinical outcome of unrelated umbilical cord blood transplant. Bone Marrow Transplant 40:201–208
Acknowledgement
This work was supported by grants from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (0520290) and from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare and Family affairs, Republic of Korea (A070001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hyoung Jin Kang and Keon Hee Yoo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kang, H.J., Yoo, K.H., Lee, J.W. et al. Double umbilical cord blood transplantation for children and adolescents. Ann Hematol 89, 1035–1044 (2010). https://doi.org/10.1007/s00277-010-0985-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-010-0985-2