Abstract
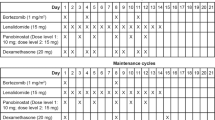
Bortezomib synergizes with melphalan in preclinical and early clinical studies. Updated data from our phase 1/2 study assessing the safety and efficacy of bortezomib plus melphalan in relapsed/refractory multiple myeloma (MM) are presented. Bortezomib (0.7, 1.0, or 1.3 mg/m2) on days 1, 4, 8, and 11 and oral melphalan (0.025–0.25 mg/kg) on days 1–4 of a 28-day cycle were administered. Hematologic toxicities defined the maximum tolerated dose as bortezomib 1.0 mg/m2 and melphalan 0.10 mg/kg. Because dose-limiting toxicities were attributed to the more myelosuppressive melphalan, cohorts 9 and 10 with higher bortezomib (1.3 mg/m2) and lower melphalan (0.025 and 0.10 mg/kg) doses were added. Responses occurred in 32/46 (70%) evaluable patients: two complete (4%), five near-complete (11%), 16 partial (35%), and nine minimal (20%). Complete and near-complete responses were observed only with higher bortezomib doses. Response rates were similar in patients with prior melphalan or bortezomib. Median progression-free survival was 9 months (range, 1–24), and overall survival was 32 months (range, 1–54). The most common grade 3/4 hematologic adverse events (AEs) were neutropenia (31%/0%), thrombocytopenia (25%/2%), and anemia (13%/0%). Grade 4 tumor lysis syndrome was reported in one patient. Fewer grade 3/4 hematologic AEs were reported in cohorts 9 and 10 than in cohorts receiving lower bortezomib and higher melphalan doses. In conclusion, bortezomib plus melphalan is a steroid- and immunomodulatory drug-free regimen that may provide a treatment alternative for elderly patients and patients with significant comorbidity.

Similar content being viewed by others
References
Anderson KC, Alsina M, Bensinger W, Biermann JS, Chanan-Khan A, Comenzo RL, de Castro CM, Djulbegovic B, Farag S, Huff CA, Meredith R, Schriber J, Shrieve D, Singhal S, Smith MR, Stockerl-Goldstein K, Vose JM, Weber D, Yahalom J, Yunus F (2007) Multiple myeloma. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 5:118–147
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78:21–33
Jawed I, Lee C, Tward JD, Macdonald OK, Martnicic D, Vudarla N (2007) Survival outcomes for multiple myeloma over three decades: A Surveillance, Epidemiology, and End Results (SEER) analysis. Presented at American Society of Clinical Oncology Annual Meeting, June 1–5, 2007, Chicago, IL, USA. Abstract 8019
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875–1883
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R (1996) A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 335:91–97
Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, Lauta VM, Bergonzi C, Barbui A, Caravita T, Capaldi A, Pregno P, Guglielmelli T, Grasso M, Callea V, Bertola A, Cavallo F, Falco P, Rus C, Massaia M, Mandelli F, Carella AM, Pogliani E, Liberati AM, Dammacco F, Ciccone G, Boccadoro M (2004) Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood 104:3052–3057
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348:2609–2617
Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J (2005) Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol 129:776–783
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352:2487–2498
Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San Miguel J, Blade J, Boccadoro M, Cavenagh J, Alsina M, Rajkumar SV, Lacy M, Jakubowiak A, Dalton W, Boral A, Esseltine DL, Schenkein D, Anderson KC (2007) Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 110:3557–3560
Richardson PG, Mitsiades C, Ghobrial I, Anderson K (2006) Beyond single-agent bortezomib: combination regimens in relapsed multiple myeloma. Curr Opin Oncol 18:598–608
Ma MH, Yang HH, Parker K, Manyak S, Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E, Neeser J, Mikail A, Adams J, Sjak-Shie N, Vescio RA, Berenson JR (2003) The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res 9:1136–1144
Berenson JR, Yang HH, Sadler K, Jarutirasarn SG, Vescio RA, Mapes R, Purner M, Lee SP, Wilson J, Morrison B, Adams J, Schenkein D, Swift R (2006) Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol 24:937–944
National Cancer Institute (1999) Common toxicity criteria manual: common txicity Criteria v2.0. http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf
National Cancer Institute (2003) Common terminology criteria for adverse events v3.0 (CTCAE). http://ctep.cancer.gov/forms/CTCAE_Index.pdf
Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 102:1115–1123
Millennium Pharmaceuticals (2006) Velcade (bortezomib) for Injection [package insert]. Millennium Pharmaceuticals, Cambridge, MA
Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Schlossman R, Munshi NC, Hideshima T, Anderson KC (2003) The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood 101:2377–2380
Berenson JR, Jagannath S, Barlogie B, Siegel DT, Alexanian R, Richardson PG, Irwin D, Alsina M, Rajkumar SV, Srkalovic G, Singhal S, Limentani S, Niesvizky R, Esseltine DL, Trehu E, Schenkein DP, Anderson K (2005) Safety of prolonged therapy with bortezomib in relapsed or refractory multiple myeloma. Cancer 104:2141–2148
Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA (2006) Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 24:3113–3120
Palumbo A, Ambrosini MT, Benevolo G, Pregno P, Pescosta N, Callea V, Cangialosi C, Caravita T, Morabito F, Musto P, Bringhen S, Falco P, Avonto I, Cavallo F, Boccadoro M (2007) Bortezomib, melphalan, prednisone and thalidomide for relapsed multiple myeloma. Blood 109:2767–2772
Richardson PG, Hideshima T, Mitsiades C, Anderson KC (2007) The emerging role of novel therapies for the treatment of relapsed myeloma. J Natl Compr Canc Netw 5:149–162
Manochakian R, Miller KC, Chanan-Khan AA (2007) Clinical impact of bortezomib in frontline regimens for patients with multiple myeloma. Oncologist 12:978–990
Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, Diaz-Mediavilla J, Lahuerta JJ, de la Rubia J, Terol MJ, Sureda A, Bargay J, Ribas P, de Arriba F, Alegre A, Oriol A, Carrera D, Garcia-Larana J, Garcia-Sanz R, Blade J, Prosper F, Mateo G, Esseltine DL, van de Velde H, San Miguel JF (2006) Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood 108:2165–2172
Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K (2007) New drugs for myeloma. Oncologist 12:664–689
San Miguel JF, Schlag R, Khuageva N, Shpilberg O, Dimopoulos M, Kropff M, Spicka I, Petrucci M, Samoilova O, Dmoszynska A, Abdulkadyrov K, Schots R, Jiang B, Palumbo A, Mateos M, Liu K, Cakana A, van de Velde H, Richardson P (2007) A phase 3 study comparing BortezomibMelphalanPrednisone (VMP) with MelphalanPrednisone (MP) in newly diagnosed multiple myeloma. Presented at American Society of Hematology Annual Meeting, November 16, 2007, Atlanta, GA, USA. Abstract 76
Berenson JR, Yellin O, Woytowitz D, Flam MS, Cartmell A, Patel R, Duvivier H, Nassir Y, Swift RA (2007) Bortezomib, ascorbic acid and melphalan (BAM) therapy for patients (pts) with newly diagnosed multiple myeloma (MM): an effective and well-tolerated frontline regimen. Presented at American Society of Hematology Annual Meeting, November 16, 2007, Atlanta, GA, USA. Abstract 3602
Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, Garcia P, Lahuerta JJ, de la Rubia J, Terol MJ, Sureda A, Bargay J, Ribas P, de Arriba F, Alegre A, Oriol A, Carrera D, Garcia-Larana J, Garcia-Sanz R, Blade J, Prosper F, Mateo G, Esseltine D, van de Velde H, San Miguel J (2007) Frontline VMP in elderly untreated multiple myeloma patients: extended follow-up. Presented at XIth International Myeloma Workshop, June 25, 2007, Kos Island, Greece. Poster PO-718
Acknowledgments
The authors thank Ingrid Koo, PhD, and Bless Castro, PhD, of Helix Medical Communications LLC for providing medical writing support on behalf of Millennium Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berenson, J.R., Yang, H.H., Vescio, R.A. et al. Safety and efficacy of bortezomib and melphalan combination in patients with relapsed or refractory multiple myeloma: updated results of a phase 1/2 study after longer follow-up. Ann Hematol 87, 623–631 (2008). https://doi.org/10.1007/s00277-008-0501-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-008-0501-0