Abstract
Von Willebrand disease (VWD), the most common hereditary bleeding disorder, is divided into three types depending on the quantitative (type 1 and 3) or qualitative (type 2) abnormality of von Willebrand factor (VWF). About 70–80% of VWD patients can be treated with the synthetic product desmopressin, while the others necessitate factor VIII/VWF concentrates. In addition to the treatment of bleeding episodes, therapeutic regimens include short- or long-term prophylaxis. While the literature data on short-term prophylaxis in VWD are consistent and clearly show the safety and efficacy of such a therapeutic approach, little evidence is available regarding long-term prophylaxis, and although the preliminary results are encouraging, they need to be validated by large prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Von Willebrand disease (VWD), the most common hereditary bleeding disorder, results from a quantitative or a qualitative abnormality of von Willebrand factor (VWF) [1–3]. Inherited VWD has been subdivided into three types: In types 1 and 3, VWD reflect, respectively, the partial or virtually complete deficiency of VWF, while in type 2, VWD reflects a qualitative deficiency of VWF. Qualitative VWD type 2 is further divided into four variants: 2A, 2B, 2M, and 2N, based on the characteristics of dysfunctional VWF. Type 1 is the most common form of VWD, accounting for approximately 80% of all cases, and it is usually characterized by relatively mild bleeding tendency that primarily involves mucosal bleeding [4, 5]. Mucocutaneous bleeding symptoms are also frequent in patients with type 2 VWD, which affects approximately 20% of VWD patients. By contrast, patients with the rare type 3 VWD (1–3% of all cases) have a more severe disease, complicated also by hemarthroses and muscle hematomas as observed in severe hemophilia.
The treatment of VWD is based on the type of the disease: In fact, while desmopressin (DDAVP) is the treatment of choice for most patients with type 1 VWD, it is ineffective in patients with the more severe type 3 and in most patients with type 2 disease where it is necessary to use VWF-containing factor VIII (FVIII) products [6]. Moreover, responsive patients treated with repeated DDAVP infusions given to maintain hemostasis (e.g., after surgery or invasive procedures) may became unresponsive (phenomenon know as “tachyphylaxis”). Thus, in approximately 20–30% of VWD patients, the replacement of VWF and FVIII with plasma-derived concentrates is the mainstay of treatment [7, 8].
Patients with VWD may require short- or long-term prophylaxis treatment. Short-term prophylaxis is usually performed to prevent excessive bleeding after surgery or invasive procedures, while long-term prophylaxis may be needed to control recurrent mucosal and joint bleeding complicating the more severe forms of VWD. These two latter therapeutic options will be summarized in this review.
Search strategy
An electronic search on MEDLINE and EMBASE without temporal limits was performed using different combinations of the following keywords: “von Willebrand disease,” “VWD,” “von Willebrand factor,” “VWF,” “short-term prophylaxis,” “long-term prophylaxis,” “prevention,” “bleeding,” “hemorrhage,” “gastrointestinal bleeding,” “plasma-derived concentrates,” “clotting factor concentrates,” “desmopressin,” “DDAVP,” “treatment,” “therapy,” “surgery,” “invasive procedures,” “Haemate/Humate-P,” “Fanhdi,” and “Alphanate.” In addition, the bibliographic references of all retrieved studies and reviews were assessed for additional reports of clinical trials. Unpublished works were identified by searching the abstract books of the most important congresses on this topic (International Society of Thrombosis and Haemostasis and World Federation of Hemophilia).
Short-term prophylaxis in von Willebrand disease
There are several reports in the literature on the use of DDAVP or FVIII/VWF concentrates as prophylaxis of hemorrhages during surgery or invasive procedures. However, because of the onset of tachyphylaxis after repeated DDAVP infusions, most of the major surgical procedures are managed with plasma-derived concentrates. FVIII/VWF concentrates licensed in Italy for the treatment of VWD are Haemate P (CSL Behring), Alphanate (Grifols), Fanhdi (Grifols), and Immunate (Baxter).
Federici et al. [9] retrospectively collected data from 63 cases of oral surgery in VWD patients and found that local therapy with tranexamic acid and fibrin glue with DDAVP, given subcutaneously at a single dose of 0.3 μg/kg before surgery, can prevent bleeding complication in the majority (82%) of patients with VWD. Leissinger et al. [10] found that high-dose DDAVP intranasal spray was effective in 93% of cases in preventing bleeding in 333 patients with mild hemophilia A or mild/moderate type 1 VWD (172 patients).
In a single-center 10-year retrospective review conducted in UK, Nitu-Whalley et al. [11] identified 27 patients treated with DDAVP for 35 surgical events and 38 patients who received intermediate-purity FVIII/VWF concentrates for 68 elective surgical events. DDAVP was administered in a dose of 0.3 μg/kg intravenously over 30 min. As regard to plasma-derived concentrates, for major surgery, the median pre- and postoperative doses were 54 and 43 IU/kg, respectively, and for minor surgery, the median doses varied between 34 and 52 IU/kg preoperatively and between 27 and 37 IU/kg postoperatively. The effectiveness of hemostasis was excellent in 32 events (91%) treated with DDAVP and in 56 events (82%) treated with concentrates.
The intermediate-purity FVIII/VWF concentrate Haemate P was utilized in a large retrospective study organized by the Canadian Hemophilia Centers. Patients were treated for 437 events, including bleeding episodes and surgical procedures. The rates of excellent-to-good responses were 97% overall and 99% in surgical procedures. The median dose of concentrate per infusion used to treat surgical events was 69.1 IU VWF:RCo/kg (range 11.9–222.8) [12].
We have reported the experience of three Italian hemophilia centers on 26 VWD patients who underwent 43 surgical or invasive procedures (14 major surgery, 11 minor surgery, 11 dental extractions, and seven invasive diagnostic procedures) under coverage with Haemate P [13]. The mean daily dose of the concentrate given was 39.3 IU VWF:RCo/kg (range 25–52.5) for major surgery, 28.7 IU VWF:RCo/kg (range 21.4–34.8) for minor surgery, 24.0 IU VWF:RCo/kg (range 23.5–25) for dental extractions, and 32.3 IU VWF:RCo/kg (range 27.3–37) for invasive procedures. The mean days of treatment were 9.7 (range 5–23) for major surgery, 4.2 (range 2–7) for minor surgery, 1.6 (range 1–5) for dental extractions, and 2.7 (range 1–5) for invasive procedures. As only a bleeding episode was recorded without drug-related adverse events, we concluded that Haemate P was safe and effective in preventing excessive bleeding after major and minor surgery or invasive procedures in VWD patients.
More recently, an open-label prospective study collected data from 39 subjects undergoing 42 urgent surgical treatment events [14]. The median loading dose based upon VWF:RCo activity was 82.3 IU/kg (range 32.5–216.8), and the median maintenance dose per infusion was 52.8 IU kg (range 24.2–196.5) for a median of 3 days (range 1–50 days). The median number of infusions per event was 6 (range 1–67 infusions). Bernstein et al. [15] enrolled 35 VWD patients who received Haemate P as prophylaxis of excessive bleeding during elective surgery (25 major, seven minor, and three oral surgery) and found an effective hemostasis in 91.4% of the cases. An Italian retrospective multicenter cohort study [16] evaluated the response to Haemate P in 100 VWD patients treated for bleeding, surgeries, or prophylaxis and recorded a 97% of excellent/good clinical response in the 56 patients who underwent 73 surgical or invasive procedures.
Finally, a prospective multicenter trial on 29 subjects with VWD undergoing elective surgery found that Haemate P, whose preoperative loading dose was based on a pharmacokinetic study, provided an excellent or good hemostasis in 96.3% of subjects on the day of surgery and 100% on the following days [17].
The highly purified, doubly virus-inactivated FVIII/VWF concentrate Fanhdi has been reported to be efficacious in the management of VWD in a retrospective clinical study [18]. In 22 patients, 12 bleeding episodes and 14 invasive procedures were treated with Fanhdi. There was 93% excellent or good efficacy and no adverse events.
The results of a large international prospective study by the Alphanate study Group were published in 2002 and included 39 patients receiving prophylactic treatment for 71 surgical or invasive diagnostic procedures. The median number of infusion per procedure was 3, and the dosages for the first and subsequent infusions were 60 and 40 IU/kg VWF:RCo, respectively. A good clinical response with this FVIII/VWF concentrate was observed in 96% of patients [19]. In this study, it was also demonstrated that surgery could be safely undertaken even when Alphanate did not correct the bleeding time.
Goudemand et al. [20] reported the French clinical experience on the use of the very-high-purity VHP VWF concentrate in 75 VWD patients treated on 99 occasions either to control spontaneous bleeding or to prevent hemorrhagic risk associated with surgery. Successful treatment of 31 minor and 23 major surgical procedures was reported.
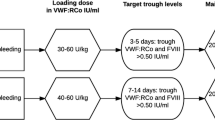
As a whole, the literature results (Table 1) document that 20–50 IU/kg of FVIII/VWF concentrates given once daily until healing is complete are hemostatically effective in preventing bleeding in more than 90% of surgical or invasive procedures. Thus, for major procedures, FVIII and VWF:RCo levels should be raised to 80–100 UI/dl at the time of surgery and maintained above 50 IU/dl for at least 7–14 days. For minor procedures, FVIII and VWF:RCo levels above 50 IU/dl at the time of surgery are advisable, followed by levels above 30 IU/dl for at least 5–7 days. Finally, dental extractions or invasive procedures may be managed with a single concentrate infusion aimed to reach VWF:RCo/FVIII levels of about 50 IU/dl [21, 22].
However, the accumulation of FVIII that is exogenously infused together with that endogenously synthesized and stabilized by the infused VWF may result in very high FVIII concentrations (>150 IU/dl) when several infusions are given for severe bleeding episodes or to cover major surgery. There is some concern that sustained high concentrations of FVIII may increase the risk of postoperative deep-vein thrombosis, as suggested by recent observations [23]. Therefore we suggest, when repeated injections of FVIII/VWF concentrates are administered, to monitor daily FVIII plasma levels. To avoid high postinfusion FVIII plasma levels, a high-purity VWF concentrate with low FVIII content (Wilfactin, LFB) has been recently developed, and its efficacy has been tested in a prospective study on 50 VWD patients [24]. The hemostatic outcome was judged excellent or good in all the 108 surgical or invasive procedures performed, and no adverse thrombotic complications were recorded.
Long-term prophylaxis in von Willebrand disease
Patients with more severe forms of VWD may have recurrent episodes of mucocutaneous bleeding and also hemarthrosis and hematomas and thus are candidate for long-term secondary prophylaxis [25, 26]. However, whereas prophylaxis in hemophilia has become widespread in recent years, this mode of treatment appears to be practiced only occasionally in VWD patients, and literature data are scarce [27, 28].
An Italian cohort study [29] on 452 VWD patients regularly followed up included 11 patients in a long-term prophylaxis program because of frequent recurrence of bleeds at the same sites. Prophylaxis was started because of gastrointestinal bleeds in seven patients (one type 3, two type 2A, one type 2M, and one type 1) and for joint bleeds in four type 3 patients. Effectiveness of prophylaxis was based on resolution/reduction in bleeding as well as on the number of transfused red blood cells (RBC) and days of hospitalization. Prophylaxis stopped bleeding in eight patients and largely reduced hospitalization and RBC transfusions in the remaining three. When prophylaxis was compared with previous on-demand regimen in all 11 cases, the annual total FVIII IU of concentrate as well as the number of RBC used and days of hospitalization were significantly reduced.
Coppola et al. [30] reported the benefit of a long-term prophylaxis regimen with Haemate P, given at a dose of 40 IU/kg thrice weekly, in a patient with type 3 VWD and recurrent severe gastrointestinal bleeding because of multiple vascular mucosal abnormalities. Similarly, we reported the efficacy of Haemate P (2,000 IU on alternate days) as prophylaxis of recurrent bleeding from a malignant esophageal ulcer [31].
The largest experience regarding secondary long-term prophylaxis has been collected in Sweden in 37 patients with severe forms of VWD (three type 1, three type 2, three type 2B, and 28 type 3) [27]. The median age was 33 years with patients starting prophylaxis at the age of 13 years. Prophylactic treatment was defined as at least one infusion per week for 45 weeks annually. The main indications for prophylaxis were joint bleeds or nose/mouth bleeds, and some patients required prophylaxis for menorrhagia or gastrointestinal bleeding. The doses used for prophylaxis ranged from 12 to 50 IU of FVIII/kg (mean 24 IU). The injection frequency ranged from 1 to 3 times weekly, depending on the bleeding pattern and response to treatment. The median duration of prophylaxis was 11 years, and most patients continued indefinitely. The authors found that the number of bleeds was reduced dramatically during prophylaxis. Another important observation of the study was that children who started prophylaxis because of nose or mouth bleeds never reported joint bleeds or developed signs of arthropathy. There were no reports of thrombosis.
The importance of prophylaxis in preventing arthropathy was recently outlined by Traivaree et al. [32] who shifted four type 3 VWD children with severe musculoskeletal bleeds from on-demand treatment to a regular long-term prophylaxis (55 UI/kg weekly or twice weekly).
Finally, a retrospective survey of data records on VWD patients organized among ten Italian hemophilia centers collected 17 cases of long-term secondary prophylaxis with Haemate P performed to prevent recurrent bleeding at the same site (47% in the gastrointestinal tract and 35% in joints) [16]. The FVIII/VWF concentrate was given three times (53% of cases) or twice (47%) a week, with clinical responses rated as excellent/good in 100% of cases. No serious adverse events, including thrombosis, were reported.
Conclusions
While the literature data document that short-term prophylaxis in von Willebrand disease is safe and effective, preliminary data on long-term prophylaxis are encouraging, but large prospective studies must be performed before detailed recommendations can be made.
In fact, several issues remain to be addressed regarding the long-term prophylaxis in VWD such as dosing, cost-effectiveness of this regimen vs on-demand regimen, and impact on quality of life. To address these issues, an international study, the von Willebrand Disease Prophylaxis Network (vWD PN), has been initiated [33].
References
Rodeghiero F, Castaman G, Dini E (1987) Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood 69:454–459
Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, Mazurier C, Meyer D, Nichols WL, Nishino M, Peake IR, Rodeghiero F, Schneppenheim R, Ruggeri ZM, Srivastava A, Montgomery RR, Federici AB (2006) Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost 4:2103–2114
Federici AB (2004) Clinical diagnosis of von Willebrand disease. Haemophilia 10(Suppl 4):169–176
Federici AB, Castaman G, Mannucci PM (2002) Guidelines for the diagnosis and management of von Willebrand disease in Italy. Haemophilia 8:607–621
Franchini M, Lippi G (2007) The role of von Willebrand factor in hemorrhagic and thrombotic disorders. Crit Rev Clin Lab Sci 44:115–149
Mannnucci PM (2001) How I treat patients with von Willebrand disease. Blood 97:1915–1919
Mannucci PM (2000) Desmopressin (DDAVP) in the treatment of bleeding disorders: the first twenty years. Haemophilia 6(Suppl 1):60–67
Mannucci PM (2004) Treatment of von Willebrand’s disease. N Engl J Med 351:683–694
Federici AB, Sacco R, Stabile F, Carpenedo M, Zingaro E, Mannucci PM (2000) Optimising local therapy during oral surgery in patients with von Willebrand disease: effective results from a retrospective analysis of 63 cases. Haemophilia 6:71–77
Leissinger C, Becton D, Cornell C Jr, Cox Gill J (2001) High-dose DDAVP intranasal spray (Stimate) for the prevention and treatment of bleeding in patients with mild haemophilia A, mild or moderate type 1 von Willebrand disease and symptomatic carriers of haemophilia A. Haemophilia 7:258–266
Nitu-Whalley IC, Griffioen A, Harrington C, Lee CA (2001) Retrospective review of the management of elective surgery with desmopressin and clotting factor concentrates in patients with von Willebrand disease. Am J Hematol 66:280–284
Lillicrap D, Poon M-C, Walker I, Xie F, Schwartz BA (2002) Efficacy and safety of the factor VIII/von Willebrand Factor concentrate, Haemate-P/Humate-P: ristocetin cofactor unit dosing in patients with von Willebrand disease. Thromb Haemost 87:224–230
Franchini M, Rossetti G, Tagliaferri A, Pattacini C, Pozzoli D, Lippi G, Manzato F, Bertuzzo D, Gandini G (2003) Efficacy and safety of factor VIII/von Willebrand factor concentrate (Haemate-P) in preventing bleeding during surgery or invasive procedures in patients with von Willebrand’s disease. Haematologica 88:1279–1283
Thompson AR, Gill JC, Ewenstein BM, Mueller-Velten G, Schwartz BA (2004) Successful treatment for patients with von Willebrand disease undergoing urgent surgery using factor VIII/von Willebrand factor concentrate (Humate-P). Haemophilia 10:42–51
Bernstein J, Cox Gill J, Leissinger CA, Di Paola J, Ragni MV, Shopnick R, Valentino RA, Friedman C, Knaub S (2006) Safety and efficacy of von Willebrand factor/factor VIII concentrate (Humate-P) for prophylaxis of excessive bleeding during elective surgery in patients with von Willebrand disease. Blood 108:4076 Abstract
Federici AB, Castaman G, Franchini M, Morfini M, Zanon E, Coppola A, Tagliaferri A, Boeri E, Mazzucconi MG, Rossetti G, Mannucci PM (2007) Clinical use of Haemate P in inherited von Willebrand disease: a cohort study on 100 Italian patients. Haematologica 92:944–951
Lethagen S, Kyrle PA, Castaman G, Haertel S, Mannucci PM; the Haemate P Surgical Study Group (2007) Von Willebrand factor/factor VIII concentrate (Haemate P) dosing based on pharmacokinetics: a prospective multicenter trial in elective surgery. J Thromb Haemost 5:1420–1430
Federici AB, Baudo F, Caracciolo C, Mancuso G, Mazzucconi MG, Musso R, Schinco PC, Targhetta R, Mannucci PM (2002) Clinical efficacy of highly purified, doubly virus-inactivated factor VIII/von Willebrand factor concentrate (Fanhdi) in the treatment of von Willebrand disease: a retrospective clinical study. Haemophilia 8:761–767
Mannucci PM, Chediak J, Hanna W, Byrnes J, Marlies L, Ewenstein BM (2002) Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood 99:450–456
Goudemand J, Negrier C, Ounnoughene N, Sultan Y (1998) Clinical management of patients with von Willebrand’s disease with a VHP vWF concentrate: the French experience. Haemophilia 4(Suppl 3):48–52
Ewenstein BM (2001) Use of ristocetin cofactor activity in the management of von Willebrand disease. Haemophilia 7(Suppl 1):10–15
Laffan M, Brown SA, Collins PW, Cumming AM, Hill FG, Keeling D, Peake IR, Pasi KJ (2004) The diagnosis of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors’ Organization. Haemophilia 10:199–217
Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, Weltermann A, Speiser W, Lechner K, Eichinger S (2000) High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med 343:457–462
Borel-Derlon A, Federici AB, Roussel-Robert V, Goudemand J, Lee CA, Scharrer I, Rothschild C, Berntorp E, Henriet C, Tellier Z, Bridey F, Mannucci PM (2007) Treatment of severe von Willebrand disease with a high-purity von Willebrand factor concentrate (Wilfactin): a prospective study of 50 patients. J Thromb Haemost 5:1115–1124
Abshire TC (2006) Prophylaxis and von Willebrand’s disease. Thromb Res 118S1:S3–S7
Makris M (2006) Gastrointestinal bleeding in von Willebrand disease. Thromb Res 118S1:S13–S17
Lethagen S (2006) Clinical experience of prophylactic treatment in von Willebrand disease. Thromb Res 118S1:S9–S11
Berntorp E, Petrini P (2005) Long-term prophylaxis in von Willebrand disease. Blood Coagul Fibrinolysis 16(Suppl 1):S23–S26
Federici AB, Gianniello F, Canciani MT, Mannucci PM (2005) Secondary long-term prophylaxis in severe patients with von Willebrand’s disease: an Italian cohort study. Blood 106:507a Abstract 1782
Coppola A, Cimino E, Conca P, Simone C, Tufano A, Tarantino G, Cerbone AM, Minno G (2006) Long-term prophylaxis with intermediate-purity factor VIII concentrate (Haemate P) in a patient with type 3 von Willebrand disease and recurrent gastrointestinal bleeding. Haemophilia 12:90–94
Franchini M, Giuffrida A, Gandini G (2005) Efficacy of Haemate-P as prophylaxis of recurrent bleeding in a patient with type 2B von Willebrand's disease. Blood Coagul Fibrinolysis 16:571–572
Traivaree C, Blanchette V, Hilliard P, Stain A, Floros G, Carcao M (2006) Type 3 VWD: clinical manifestations and the need for prophylaxis. Haemophilia 12(Suppl 2):918 Abstract
Berntorp E, Abshire T (2006) The von Willebrand Disease prophylaxis network (vWD PN): exploring a treatment concept. Thromb Res 118S1:S19–S22
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Franchini, M., Targher, G. & Lippi, G. Prophylaxis in von Willebrand disease. Ann Hematol 86, 699–704 (2007). https://doi.org/10.1007/s00277-007-0343-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-007-0343-1