Abstract
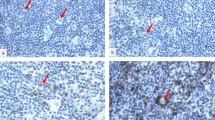
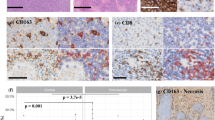
This study aimed to assess the differences in the cellular composition of the inflammatory reactive background around tumoral cells of classical Hodgkin’s lymphomas (cHL) inside and outside the HIV settings. This retrospective study evaluates the infiltrating T lymphocytes (CD4 and CD8), natural killer cells (CD57+ cells), and more especially cytotoxic cells [granzyme B (GrB) and TIA-1+ cells] in the background of 99 EBV+ cHL. Sections from paraffin-embedded tumor samples from nine HIV-infected cHL patients were immunostained, using standard immunohistochemical protocols and were compared to a control group of 90 HIV-noninfected cHL patients. Our clinical and histological data indicate that HIV-infected cHL patients present a higher frequency of mixed cellularity (MC) histological subtypes, more advanced disease stages, a poor response to treatment, and a poor overall survival compared to control patients. In controls, CD4/CD8 and GrB/TIA-1 ratios were determined as 2:1 and 1:2, respectively. The inflammatory infiltrate of HIV-infected patients had a significant reduction of CD4+ T lymphocytes (CD4/CD8 ratio 1:23), a decrease in infiltrating GrB+ cells (activated cytotoxic cells) and an increase in infiltrating TIA+ T cells (mainly nonactivated cytotoxic cells) in these patients (GrB/TIA-1 ratio 1:12). In conclusion, this study highlights an important intratumoral loss of CD4+ T cells (striking inversion in the CD4/CD8 ratio) and a decrease in intratumoral activated cytotoxic T lymphocytes in HIV-associated cHL patients. Further studies are required to confirm these results and to determine the role of these findings on the antitumoral immune response observed in HIV-associated cHL.

Similar content being viewed by others
References
Alos L, Navarrete P, Morente V, Garcia F, Garrido M, Plana M, Mozos A, Lopez A, Gil C, Pumarola T, Caballero M, Blanch JL, Fumero E, Miro JM, Gallart T, Gatell JM, Campo E (2004) Immunoarchitecture of lymphoid tissue in HIV-infection during antiretroviral therapy correlates with viral persistence. Mod Path 18:127–136
Álvaro T, Lejeune M, Salvadó MT, Bosch R, García JF, Jaén J, Banham AH, Roncador G, Montalbán C, Piris MA (2005) Outcome in Hodgkin Lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T-cells. Clin Cancer Res 11:1467–1473
Ames ED, Conjalka MS, Goldberg AF, Hirschman R, Jain S, Distenfeld A, Metroka CE (1991) Hodgkin’s disease and AIDS. Twenty-three new cases and a review of the literature. Hematol/Oncol Clin North Am 5:343–356
Anderson P, Nagler-Anderson C, O’Brien C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF (1990) A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol 144:574–582
Andersson J, Kinloch S, Sonnerborg A, Nilsson J, Fehniger TE, Spetz AL, Behbahani H, Goh LE, McDade H, Gazzard B, Stellbrink H, Cooper D, Perrin L (2002) Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J Infect Dis 185:1355–1358
Bellas C, Santon A, Manzanal A, Campo E, Martin C, Acevedo A, Varona C, Forteza J, Morente M, Montalban C (1996) Pathological, immunological, and molecular features of Hodgkin’s disease associated with HIV infection. Comparison with ordinary Hodgkin’s disease. Am J Surg Pathol 20:1520–1524
Benito JM, Lopez M, Soriano V (2004) The role of CD8+ T-cell response in HIV infection. AIDS Rev 6:79–88
Bladergroen BA, Meijer CJ, ten Berge RL, Hack CE, Muris JJ, Dukers DF, Chott A, Kazama Y, Oudejans JJ, van Berkum O, Kummer JA (2002) Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with non-Hodgkin and Hodgkin lymphoma: a novel protective mechanism for tumor cells to circumvent the immune system? Blood 99:232–237
Bofill M, Gombert W, Borthwick NJ, Akbar AN, McLaughlin JE, Lee CA, Johnson MA, Pinching AJ, Janossy G (1995) Presence of CD3+CD8+Bcl-2(low) lymphocytes undergoing apoptosis and activated macrophages in lymph nodes of HIV-1+ patients. Am J Pathol 146:1542–1555
Calza L, Manfredi R, Colangeli V, Dentale N, Chiodo F (2003) Hodgkin’s disease in the setting of human immunodeficiency virus infection. Scand J Infect Dis 35:136–141
Camilleri-Broet S, Ferme C, Berger F, Lepage E, Bain S, Briere J, Marmey B, Gaulard P, Audouin J (2004) TiA1 in advanced-stage classical Hodgkin’s lymphoma: no prognostic impact for positive tumour cells or number of cytotoxic cells. Virchows Arch 445:344–346
Dolcetti R, Boiocchi M, Gloghini A, Carbone A (2001) Pathogenetic and histogenetic features of HIV-associated Hodgkin’s disease. Eur J Cancer 37:1276–1287
Garcia JF, Camacho FI, Morente M, Fraga M, Montalban C, Alvaro T, Bellas C, Castano A, Diez A, Flores T, Martin C, Martinez MA, Mazorra F, Menarguez J, Mestre MJ, Mollejo M, Saez AI, Sanchez L, Piris MA (2003) Hodgkin and Reed–Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood 101:681–689
Glaser SL, Clarke CA, Gulley ML, Craig FE, DiGiuseppe JA, Dorfman RF, Mann RB, Ambinder RF (2003) Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer 98:300–309
Gold JE, Altarac D, Ree HJ, Khan A, Sordillo PP, Zalusky R (1991) HIV-associated Hodgkin disease: a clinical study of 18 cases and review of the literature. Am J Hematol 36:93–99
Graubert TA, Ley TJ (1996) How do lymphocytes kill tumor cells? Clin Cancer Res 2:785–789
Herling M, Rassidakis GZ, Medeiros LJ, Vassilakopoulos TP, Kliche KO, Nadali G, Viviani S, Bonfante V, Giardini R, Chilosi M, Kittas C, Gianni AM, Bonadonna G, Pizzolo G, Pangalis GA, Cabanillas F, Sarris AH (2003) Expression of Epstein–Barr virus latent membrane protein-1 in Hodgkin and Reed–Sternberg cells of classical Hodgkin’s lymphoma: associations with presenting features, serum interleukin 10 levels, and clinical outcome. Clin Cancer Res 9:2114–2120
Kanavaros P, Vlychou M, Stefanaki K, Rontogianni D, Gaulard P, Pantelidaki E, Zois M, Darivianaki K, Georgoulias V, Boulland ML, Gorgoulis V, Kittas C (1999) Cytotoxic protein expression in non-Hodgkin’s lymphomas and Hodgkin’s disease. Anticancer Res 19:1209–1216
Lieberman J, Shankar P, Manjunath N, Andersson J (2001) Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98:1667–1677
Liu Y, Zhuang H, Liao X, Luo X, Luo D, Cai X (2002) Immunophenotype and differential diagnosis of Hodgkin’s lymphoma. Zhonghua Xue Ye Xue Za Zhi 23:524–527
Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA (2004) Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 103:1755–1762
Montalban C, Garcia JF, Abraira V, Gonzalez-Camacho L, Morente MM, Bello JL, Conde E, Cruz MA, Garcia-Sanz R, Garcia-Larana J, Grande C, Llanos M, Martinez R, Flores E, Mendez M, Ponderos C, Rayon C, Sanchez-Godoy P, Zamora J, Piris MA (2004) Influence of biologic markers on the outcome of Hodgkin’s lymphoma: a study by the Spanish Hodgkin’s Lymphoma Study Group. J Clin Oncol 22:1664–1673
Oudejans JJ, Jiwa NM, Kummer JA, Ossenkoppele GJ, van Heerde P, Baars JW, Kluin PM, Kluin-Nelemans JC, van Diest PJ, Middeldorp JM, Meijer CJ (1997) Activated cytotoxic T cells as prognostic marker in Hodgkin’s disease. Blood 89:1376–1382
Pelstring RJ, Zellmer RB, Sulak LE, Banks PM, Clare N (1991) Hodgkin’s disease in association with human immunodeficiency virus infection. Pathologic and immunologic features. Cancer 67:1865–1873
Poppema S (2004) Regulatory T cells in Hodgkin lymphoma. Blood 103:1565–1566
Rapezzi D, Ugolini D, Ferraris AM, Racchi O, Gaetani GF (2001) Histological subtypes of Hodgkin’s disease in the setting of HIV infection. Ann Hematol 80:340–344
Re A, Casari S, Cattaneo C, Facchetti F, Cadeo G, Carosi G, Rossi G (2001) Hodgkin disease developing in patients infected by human immunodeficiency virus results in clinical features and a prognosis similar to those in patients with human immunodeficiency virus-related non-Hodgkin lymphoma. Cancer 92:2739–2745
Ree HJ, Strauchen JA, Khan AA, Gold JE, Crowley JP, Kahn H, Zalusky R (1991) Human immunodeficiency virus-associated Hodgkin’s disease. Clinicopathologic studies of 24 cases and preponderance of mixed cellularity type characterized by the occurrence of fibrohistiocytoid stromal cells. Cancer 67:1614–1621
Rodriguez JN, Aguayo DM, Martin I, Dieguez JC, Prados D, Pujol E (1994) Hodgkin’s disease and HIV: relations between CD4/CD8 rate, histology and stage. Rev Clin Esp 194:543–546
Rubio R (1994) Hodgkin’s disease associated with human immunodeficiency virus infection. A clinical study of 46 cases. Cooperative Study Group of Malignancies Associated with HIV Infection of Madrid. Cancer 73:2400–2407
Ruco LP, Di Napoli A, Pilozzi E, Talerico C, Uccella I, Giancola ML, Alba L, Antinori A (2004) Peripheral T cell lymphoma with cytotoxic phenotype: an emerging disease in HIV-infected patients? AIDS Res Hum Retrovir 20:129–133
Serrano M, Bellas C, Campo E, Ribera J, Martin C, Rubio R, Ruiz C, Ocana I, Buzon L, Yebra M et al (1990) Hodgkin’s disease in patients with antibodies to human immunodeficiency virus. A study of 22 patients. Cancer 65:2248–2254
Shresta S, MacIvor DM, Heusel JW, Russell JH, Ley TJ (1995) Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc Natl Acad Sci U S A 92:5679–5683
Skinnider BF, Mak TW (2002) The role of cytokines in classical Hodgkin lymphoma. Blood 99:4283–4297
Smyth MJ, Street SE, Trapani JA (2003) Cutting edge: granzymes A and B are not essential for perforin-mediated tumor rejection. J Immunol 171:515–518
Thompson LD, Fisher SI, Chu WS, Nelson A, Abbondanzo SL (2004) HIV-associated Hodgkin lymphoma: a clinicopathologic and immunophenotypic study of 45 cases. Am J Clin Pathol 121:727–738
Tian Q, Streuli M, Saito H, Schlossman SF, Anderson P (1991) A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 67:629–639
Tirelli U, Errante D, Dolcetti R, Gloghini A, Serraino D, Vaccher E, Franceschi S, Boiocchi M, Carbone A (1995) Hodgkin’s disease and human immunodeficiency virus infection: clinicopathologic and virologic features of 114 patients from the Italian Cooperative Group on AIDS and Tumors. J Clin Oncol 13:1758–1767
Tsimberidou AM, Sarris AH, Medeiros LJ, Mesina O, Rodriguez MA, Hagemeister FB, Romaguera J, Pro B, McLaughlin P, Dang N, Cabanillas F (2001) Hodgkin’s disease in patients infected with human immunodeficiency virus: frequency, presentation and clinical outcome. Leuk Lymphoma 41:535–544
Unger PD, Strauchen JA (1986) Hodgkin’s disease in AIDS complex patients. Report of four cases and tissue immunologic marker studies. Cancer 58:821–825
Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, Moore J, Mackay CR, Cooper DA, Saksena NK, Kelleher AD (2004) Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood 103:2238–2247
Zhang D, Shankar P, Xu Z, Harnisch B, Chen G, Lange C, Lee SJ, Valdez H, Lederman MM, Lieberman J (2003) Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood 101:226–235
Acknowledgements
The authors are grateful to all the participants of the Spanish Hodgkin’s lymphoma Study Group for their cooperation and to the National Center of Oncology Investigation (CNIO) of Madrid for the TMAs. We are also indebted to Dra. Silvia de Sanjosé (Servei d’Epidemiologia i Registre del Càncer of ICO) for her scientific revision of the manuscript and with Fernando Romeu (EOI Tortosa) for improving the text (language) of our paper.
This study was supported by grants from the Ministerio de Sanidad y Consumo, Spain (grant PI021367, grant G03/179, grant PI041467, and grant PI041440).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bosch Príncep, R., Lejeune, M., Salvadó Usach, M.T. et al. Decreased number of granzyme B+ activated CD8+ cytotoxic T lymphocytes in the inflammatory background of HIV-associated Hodgkin’s lymphoma. Ann Hematol 84, 661–666 (2005). https://doi.org/10.1007/s00277-005-1051-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-005-1051-3