Abstract
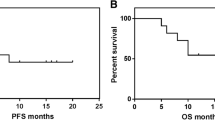
The addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) has been shown to improve the outcome in all age groups with newly diagnosed diffuse large B-cell lymphoma (DLBCL). We conducted a retrospective analysis to evaluate the impact of this combination therapy on DLBCL outcomes in Korea. From October 2001 to June 2004, newly diagnosed DLBCL patients in nine Korean institutes were included. All of these 81 patients were treated with three or more cycles of rituximab plus CHOP (R-CHOP) combination chemotherapy (R group), and followed for a minimum of 12 months. For comparison, a historical cohort of patients was used and analyzed for “Clinicopathologic characteristics of Korean non-Hodgkin’s lymphomas (NHLs) based on Revised American Lymphoma (REAL) classification” in 1999. Among the 1,098 NHL patients, the data of 214 DLBCL patients, who were treated with CHOP chemotherapy in first-line, were analyzed (C group). We compared outcomes between the C group and the R group. A total of 295 patients were evaluated (C group, 214; R group, 81). The complete response (CR) rate was higher in R group (73 vs 91%, p=0.001). The 2-year event-free survival (EFS) rate was significantly higher in R group (78 vs 85%, p=0.0194). This survival benefit was maintained in high-risk patients according to the international prognostic index (IPI) (p=0.0039), regardless of age. However, there was no significant difference in low-risk patients. The addition of rituximab to CHOP combination chemotherapy for DLBCLs showed improved outcomes, particularly in high-risk group according to the IPI. Long-term follow-up results will be needed to confirm these results.



Similar content being viewed by others
References
Chang KL, Arber DA, Weiss LM (1996) CD20: a review. Appl Immunohistochem 4:1–15
Cheson BD (2005) What is new in lymphoma? Cancer J Clin 54:260–272
Coiffier B (2005) Current strategies for the treatment of diffuse large B cell lymphoma. Curr Opin Hematol 12:259–265
Demidem A, Hanna N, Hariharan H et al (1995) Chimeric anti-CD20 antibody (IDEC C2B8) is apoptotic and sensitizes drug-resistant human B cell lymphomas to the cytotoxic effect of CDDP, VP-16 and toxins. FASEB J 9:A206
Feugier P, Van Hoof A, Sebban C et al (2005) Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. J Clin Oncol 23:4117–4126
Fisher RI, Gaynor ER, Dahlberg S et al (1993) Comparison of a standard regimen (CHOP) with three intensive chemotherpay regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 328:1002–1006
Fisher RI, Miller TP, and O’Connor OA (2004) Diffuse aggressive lymphoma. Hematology 221–236
Foran JM, Rochatiner AZS, Conningham et al (2000) European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol 18:317–324
Gordon LI, Harrington D, Andersen J et al (1992) Comparison of a second-generation combination chemotherapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin’s lymphoma. N Engl J Med 327:1342–1349
Hennessy BT, Hanrahan EO, Daly PA (2004) Non-Hodgkin’s lymphoma: an update. Lancet Oncol 5:341–353
Jaffe ES, Harris NL, Stein H et al (2001) Tumours of haematopoietic and lymphoid tissue in World Health Organization Classification of Tumors. IARC Press, Lyon, pp 171–174
Kang YK, Kim BS, Kim TW et al (1999) Clinicopathologic characteristics of Korean non-Hodgkin’s lymphomas based on REAL classification. J Korean Cancer Assoc 31:641–652
Ko YH, Kim CW, Park CS et al (1998) REAL classification of malignant lymphoma in the Republic of Korea. Cancer 83:806–812
Lee SS, Cho KJ, Kim CW, Kang YK (1999) Clinicopathological analysis of 501 non-Hodgkin’s lymphomas in Korea according to the revised European–American classification of lymphoid neoplasms. Histopathology 35:345–354
McLaughlin P, Grillo-Lopez AJ, Link BK et al (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833
Mounier N, Briere J, Gisselbrecht C et al (2003) Rituximab plus CHOP (R-CHOP) overcomes bcl-2-associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood 101:4279–4284
Pfreundschuh MG, Trumper L, Ma D et al (2004) Randomized intergroup trial of first line treatment for patients <60 years with diffuse large B-cell non-Hodgkin’s lymphoma (DLBCL) with a CHOP-like regimen with or without the anti-CD20 antibody rituximab — early stopping after the first interim analysis. J Clin Oncol 22:14S (abstract no. 6500)
Reff ME, Carner K, Chambers KS, Chinn PC et al (1994) Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83:435–445
Sehn LH, Donaldson J, Chhanabhai M et al (2005) Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23:5027–5033
Shan D, Ledbetter JA, Press OW (1998) Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood 91:1644–1652
Tobinai K, Igarashi T, Itoh K et al (2004) Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol 15:821–830
Vose JM, Link BK, Grossbard ML et al (2001) Phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J Clin Oncol 19:389–397
Wohrer S, Puspok A, Drach J et al (2004) Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) for treatment of early-stage gastric diffuse large B-cell lymphoma. Ann Oncol 15:1086–1090
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Lymphoma Study Division of the Korean Cancer Study Group., Park, Y.H., Lee, J.J. et al. Improved therapeutic outcomes of DLBCL after introduction of rituximab in Korean patients. Ann Hematol 85, 257–262 (2006). https://doi.org/10.1007/s00277-005-0060-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-005-0060-6