Abstract
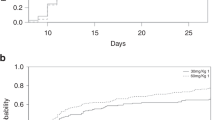
To analyze the relationship between the cellular composition of peripheral blood allografts and clinical outcome, we performed a prospective study in 45 adult patients who underwent allogeneic peripheral blood hematopoietic stem cell transplantation (HSCT) from a histocompatibility leukocyte antigen identical sibling donor for different hematological malignancies. The dose of CD34+, CD3+, CD4+, CD8+, and CD19+ lymphocytes, natural killer (NK) cells, natural killer T (NKT) cells, type 1 and type 2 dendritic cells (DC1 and DC2), as well as regulatory T (Treg) lymphocytes was analyzed. All patients were conditioned with busulphan and cyclophosphamide (BuCy2) ± VP-16 and received a short course of methotrexate and cyclosporin-A as graft-versus-host disease (GVHD) prophylaxis. Acute GVHD (aGVHD) was present in 9 of 43 (21%) patients, and chronic GVHD (cGVHD) developed in 18 of 39 (46%) patients. There was a significantly higher incidence of aGVHD in patients receiving more than 6×106/kg CD34+ cells. In univariate analysis, variables associated with better survival were as follows: a dose of less than 1.5×107/kg NKT cells and less than 1.7×106/kg DC2 for disease-free survival (DFS), and a dose of less than 3×107/kg NK cells, less than 1.5×107/kg NKT cells, less than 3×106/kg DC1, and less than 1.7×106/kg DC2 for overall survival (OS). In the Cox regression analysis, the dose of NKT cells was the only variable associated with better DFS, while the doses of NK, NKT, and CD34+ cells (less than 8×106/kg) were associated with better OS. In conclusion, different circulating cell populations, other than CD34+ cells, are also of relevance in predicting the clinical outcome after allogeneic peripheral blood HSCT.




Similar content being viewed by others
References
Little M, Storb R (2002). History of haematopoietic stem-cell transplantation. Nat Rev Cancer 2:231–238
Wagner JE, Zahurak M, Piantadosi S, Geller RB, Vogelsang GB, Wingard JR, Saral R, Griffin C, Shah N, Zehnbauer BA (1992). Bone marrow transplantation of chronic myelogenous leukemia in chronic phase: evaluation of risks and benefits. J Clin Oncol 10:779–789
Fefer A (1999) Hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ (eds) Blackwell Science, Malden, MA, pp 316–326
Vela-Ojeda J, García-Ruiz Esparza MA, Reyes-Maldonado E, Jiménez-Zamudio L et al (2004). Donor lymphocyte infusions for relapse of chronic myeloid leukemia after allogeneic stem cell transplantation: prognostic significance of the dose of CD3+ and CD4+ lymphocytes. Ann Hematol 83:295–301
Parham P, McQueen KL (2003) Alloreactive killer cells: hindrance and help for haematopoietic transplants. Nat Rev Immunol 3:108–122
Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, Jouet JP, Attal M, Bordigoni P, Cahn JY, Boiron JM, Schuller MP, Moatti JP, Michallet M (2000) Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol 18:537–546
Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, Russel N, Apperley JF, Gorin NC, Szer J, Bradstock K, Buzyn A, Clack P, Borkett K, Gratwohl A; European Group for Blood and Marrow Transplantation (2002) Transplantation of mobilized peripheral blood cells to HLA identical siblings with standard risk leukemia. Blood 100:761–767
Korbling M, Huh YO, Durett A, Mirza N, Miller P, Engel H, Anderlini P, Van Beisen K, Andreeff M, Przepiorka D, Deisseroth AB, Champlin RE (1995) Allogeneic blood stem cell transplantation: peripheralization and yield of donor derived primitive hematopoietic progenitor cells (CD34+Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood 86:2842–2848
Arat M, Arslan O, Gürman G, Dalva K, Ozcan M, Ugur A, Ilhan O (2004) The impact of granulocyte colony stimulating factor at content of donor lymphocytes collected for cellular immunotherapy. Transfus Apher Sci 30:9–15
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 15:825–828
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, Erickson K, Flowers M, Hansen J, Loughran T (1991) Chronic graft-versus host disease, other late complications of bone marrow transplantation. Semin Hematol 28:250–259
Gorin NC, Labopin M, Rocha V, Arcese W, Beksak M, Gluckman E, Ringden O, Ruutu T, Reiffers J, Bandini G, Falda M, Zikos P, Willemze R, Franssoni F; European Cooperative Group for Blood and Marrow Transplantation Acute Leukemia Working Party (2003) Marrow versus peripheral blood for geno-identical allogeneic stem cell transplantation in acute myelocytic leukemia: influence of the dose and stem cell source shows better outcome with rich marrow. Blood 102:3043–3051
Sierra J, Storer B, Hansen JA, Bjerke JW, Martin PJ, Petersdorf EW, Appelbaum FR, Bryant E, Vahauncey TR, Sale G, Sanders JE, Storb R, Sullivan KM, Anasetti C (1997) Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA matching and marrow cell dose. Blood 89:4226–4235
Zaucha JM, Gooley T, Bensinger WI, Heimfeld S, Chauncey TR, Zaucha R, Martin PJ, Flowers ME, Storek J, Georges G, Storb R, Torok-Storb B (2001) CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood 98:3221–3227
Przepiorka D, Smith TL, Folloder J, Khouri I, Ueno NT, Mehra R, Korbling M, Huh YO, Giralt S, Gajewski J, Donato M, Cleary K, Claxton D, Braunschweig I, van Besien K, Anderson BS, Anderlini P, Champlin R (1999) Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood 94:1465–1470
Heimfeld S (2003) Bone marrow transplantation: how important is CD34 cell dose in HLA-identical stem cell transplantation? Bone Marrow Transplant 17:856–858
Urbini B, Arpinati M, Bonifazi F, Chirumbolo G, Falcioni S, Stanzani M, Bandini G, Motta MR, Perone G, Giannini B, Tura S, Baccarani M, Rondelli D (2003) Allogeneic graft CD34+ cell dose correlates with dendritic cell dose and clinical outcome, but not with dendritic cell reconstitution after transplant. Exp Hematol 31:953–958
Singhal S, Powles R, Treleaven J, Kulkarni S, Sirohi B, Horton C, Millar B, Shepherd V, Tait D, Saso R, Rowland A, Long S, Mehta J (2000) A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings: should 2×106 CD34+ cells/kg be considered the minimum threshold? Bone Marrow Transplant 26:489–496
Bittencourt H, Rocha V, Chevret S, Socie G, Esperou H, Devergie A, Dal Cortivo L, Marolleau JP, Garnier F, Ribaud P, Gluckman E (2002) Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood 99:2726–2733
Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM (2002) Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100:1611–1618
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, Murphy WJ (1998) Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest 101:1835–1842
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295:2097–3010
Shilling HG, Young N, Guethlein LA, Cheng NW, Gardiner CM, Tyan D, Parham P (2002) Genetic control of human NK-cell repertoire. J Immunol 169:239–247
Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A (2002) Defective expression and function of natural killer cells triggering receptors in patients with acute myeloid leukemia. Blood 99:3661–3667
Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L Moretta A (2002) A major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res 62:6178–6186
Pascal V, Brunet C, Pradel V, Thirion X, Andre P, Faucher C, Sampol J, Dignat-George F, Blaise D, Vivier E, Chabannon C (2002) Analysis of donor NK and T cells infused in patients undergoing MHC-matched allogeneic hematopoietic transplantation. Leukemia 16:2259–2266
Yamasaki S, Henzan H, Ohno Y, Yamanaka T, Iino T, Itou Y, Kuroiwa M, Maeda M, Kawano N, Kinukawa N, Miyamoto T, Nagafuji K, Shimoda K, Inaba S, Hayashi S, Taniguchi S, Shibuya T, Gondo H, Otsuka T, Harada M; Fukuoka Blood and Marrow Transplantation Group (2003) Influence of transplanted dose of CD56+ cells on development of graft-versus-host disease in patients receiving G-CSF-mobilized peripheral blood progenitor cells from HLA-identical sibling donors. Bone Marrow Transplant 32:505–510
Miller JS, Prosper F, McCullar V (1997) Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cell collections. Blood 90:3098–3105
Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI (2002) NKT cells—conductors of tumor immunity? Curr Opin Immunol 14:165–171
Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Garcia-Ojeda M, Sibley R, Strober S (1999) Bone marrow NK1.1− and NK1.1+ T cells reciprocally regulate acute graft versus host disease. J Exp Med 189:1073–1081
Gansuvd B, Hagihara M, Yu Y, Inoue H, Ueda Y, Tsuchiya T, Masui A, Ando K, Nakamura Y, Munkhtuvshin N, Kato S, Thomas JM, Hotta T (2002) Human umbilical cord blood NK T cells kill tumors by multiple cytotoxic mechanisms. Hum Immunol 63:164–175
Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, Tokunaga K, Suzuki K, Kayagaki N, Yagita H, Hirai H, Juji T (2001) TRAIL expression by activated human CD4+ Vα24NKT cells induces in vitro and in vivo apoptosis of human myeloid leukemia cells. Blood 97:2067–2074
Linn YC, Lau LC, Kam Hui M (2002) Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. Br J Haematol 116:78–86
Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS (2001) Expansion of cytolytic CD8+ natural killer cells with limited capacity for graft-versus-host disease induction due to interferon γ production. Blood 97:2923–2931
Arpinati M, Chirumbolo G, Urbini B, Perrone G, Rondelli D, Anasetti C (2003) Role of plasmocytoid dendritic cells in immunity and tolerance after allogeneic hematopoietic stem cell transplantation. Transpl Immunol 11:345–356
Waller EK, Rosenthal H, Jones TW, Peel J, Lonial S, Langston A, Redei I, Jurickova I, Boyer MW (2001) Larger numbers of CD4bright dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood 97:2948–2956
Acknowledgements
This work was partially supported by grant no. 2004-123 from FOFOI (Fondo de Fomento a la Investigación, Dirección de Prestaciones Médicas, Coordinación de Investigación en Salud, Instituto Mexicano del Seguro Social, México).
The experiments performed in the present study complied with the current laws of Mexico and were approved by the Ethical Committee of La Raza Medical Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vela-Ojeda, J., García-Ruiz Esparza, M.A., Reyes-Maldonado, E. et al. Clinical relevance of NK, NKT, and dendritic cell dose in patients receiving G-CSF-mobilized peripheral blood allogeneic stem cell transplantation. Ann Hematol 85, 113–120 (2006). https://doi.org/10.1007/s00277-005-0037-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-005-0037-5