Abstract
Hematopoietic stem cell transplantation (HSCT) is an accepted treatment strategy for patients with severe aplastic anemia (SAA). We report our experience in a general hospital in Taiwan. From March 1985 to July 2001, 79 consecutive SAA patients, 46 male and 33 female, with a median age of 22 (4–43) years, received 80 courses of transplantation. Cyclophosphamide and total body radiation were used for the conditioning regimen, and cyclosporine-A and methotrexate for graft-versus-host disease (GVHD) prevention. Patients were followed for a median of 39 months (from 8 days to 194 months). Myeloid and platelet engraftment occurred in a median of 15 (8–27) days and 18 (8–77) days, respectively. Three patients had primary and three patients secondary graft failure. Five patients (6.8%) had grade II–IV acute GVHD in 73 evaluable patients. Chronic GVHD occurred in 23 (34.8%) patients, with extensive stage in six. Only two patients had CMV disease. The projected 3- and 5-year overall survival rates estimated by the Kaplan-Meier method were 76.08 and 74.13%, respectively. Age at transplant, non-sibling donor, mononuclear cell dose, grade II–IV acute GVHD, interval from diagnosis to transplant, and red blood cell and platelet transfusion before transplant were poor prognostic factors for overall survival by univariate analysis. Grade II–IV acute GVHD was the only prognostic factor affecting overall survival after multivariate Cox regression analysis (P=0.040). In conclusion, SAA patients receiving HSCT have good long-term survival. The low incidence of acute GVHD in our patients may be related to ethnicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hematopoietic stem cell transplantation (HSCT) is an accepted form of therapy for patients with severe aplastic anemia (SAA) who fail immunosuppression therapy. An HLA-matched sibling donor is the first consideration for transplantation [4, 6, 30]. Otherwise, patients may receive hematopoietic stem cells (HSC) from HLA-mismatched family donors [21, 40, 42] or unrelated donors [2, 3, 16, 18, 19]. Survival after transplantation has increased substantially over the past 20 years, primarily due to lower early mortality rates and better supportive care [26, 31]. The 5-year survival rates for patients receiving HLA-matched related transplants were 48%±7%, 61%±4% and 66%±6% in 1976–1980, 1981–1987 and 1988–1992, respectively [26]. The European Group for Blood and Marrow Transplantation reported 5-year survival rates of 80% after 1990 [2]. However, graft failure and graft-versus-host disease (GVHD) are still troublesome problems. Here, we evaluate the results of HSCT in 79 patients with SAA from 1985 to 2001 at our hospital in Taiwan.
Materials and methods
Patients
Between March 1985 and July 2001, 79 consecutive SAA patients received HSCT at the Taipei Veterans General Hospital in Taiwan. All patients had performance status scores of 2 or less by WHO criteria at transplantation. Most patients received HSC from their HLA-identical sibling. Others received stem cells from HLA-mismatched sibling donors, HLA-matched unrelated donors, or one of the patient’s parents.
Protocol
Patients took nystatin and vancomycin orally for gastrointestinal decontamination once they were admitted to the laminar air-flow room. The conditioning regimen consisted of cyclophosphamide 50 mg/kg/day intravenously, from day −5 to day −2 [37, 39], and total body irradiation. Intravenous mesna 100 mg/kg/day was used as uroprotection. The total body irradiation dose was 300 cGy on day −1 for matched stem cell transplants [13] or 200 cGy twice a day on day −2~−1 for mismatched or unrelated transplants [40]. Patients received granulocyte-colony stimulating factor 5 ug/kg subcutaneously beginning on day 1. Antithymocyte globulin (ATG-Fresenius) at a dose of 15 mg/kg/day on day −3~−1 was used when stem cells were from mismatched or unrelated donors. Patients received cyclosporin-A (CSA) and methotrexate (MTX) for acute GVHD prophylaxis [32]. The CSA was administered intravenously at a dose of 1.5 mg/kg every 12 h beginning on day −1, with subsequent adjustment according to the whole blood CSA level. The target CSA level was kept between 100 and 300 ng/mL. Intravenous MTX was given for 4 days, with doses of 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6 and 11. Starting 3 h after each dose of MTX, a dose of leucovorin (9 mg intravenously) was administered every 6 h over a 48-h period. CSA was shifted to oral form before discharge. Tapering of CSA started from day 100 if no acute GVHD occurred. Acute GVHD was graded 0 to IV and chronic GVHD was graded either none, limited or extensive [38]. Prednisolone was reserved for patients with acute GVHD greater than grade II. The dose was 20 mg/kg/day for 3 days, 10 mg/kg/day for 3 days, 5 mg/kg/day for 3 days, then 1 mg/kg/day for 7– 10 days until good control of GVHD was achieved. Patient with acute GVHD that was not controlled by prednisolone was given ATG-Fresenius at a dose of 10 mg/kg/day for 5–7 days.
Follow-up
The number of red blood cell transfusions and platelet transfusions before stem cell transplantation were recorded. Pretransplant tests were performed for the presence of: hepatitis B (HB)sAg, HBeAg, anti-HBS, anti-hepatitis C virus (HCV) antibody, herpes simplex virus (HSV) antibody, herpes zoster virus (HZV) antibody, cytomegalovirus (CMV) antibody, human immunodeficiency virus (HIV) antibody, and human T-lymphotropic virus-1 (HTLV-1) antibody. After transplantation, CMV surveillance (serum CMV IgM titer, blood CMV culture, urine CMV culture, and urine CMV-polymerase chain reaction [PCR]) was carried out routinely. To prevent GVHD, all of the blood products given after transplantation were irradiated before transfusion. Myeloid engraftment and platelet engraftment were defined as a neutrophil count of greater than 0.5×109/L and a platelet count of greater than 20×109/L for 3 consecutive days, respectively, and transfusion independent [14]. Patients with primary graft failure were those whose neutrophil and platelet counts never reached the above-stated levels. Secondary graft failure was defined as the loss of an engrafted transplant.
Statistical methods and data analysis
The chi-square test was used to compare categoric variables. Survival curves were estimated using the Kaplan-Meier method, with censoring at the time of last contact. The log-rank test was used to assess the significance of differences in survival for each factor (gender, age at transplant, stem cell source, mononuclear cell dose, occurrence of acute GVHD or chronic GVHD, status of HBsAg before transplant, time interval from diagnosis of SAA to transplant, number of red cell transfusions before stem cell transplant, and number of platelet transfusions before stem cell transplant). Variables with P<0.1 in the univariate analysis were included in the multivariate analysis, which used Cox regression model. All P values are two-sided and considered statistically significant if less than 0.05.
Results
Patients characteristics
From March 1985 to July 2001, 46 male and 33 female patients with SAA received 80 courses of HSCT (Table 1). One 11-year-old patient (UPN 89) received syngeneic bone marrow from her sister but had secondary graft failure 7 years later. She successfully received a second bone marrow transplant from the same donor. The age of patients at transplantation ranged from 4 to 43 years (median 22 years). The stem cell sources were syngeneic marrow in three cases, HLA-identical sibling marrow in 59 cases, HLA-mismatched sibling marrow in one, HLA-identical sibling peripheral blood in two, matched unrelated marrow in nine, and marrow from the patient’s parents in six. Overall, there were 73 HLA-matched transplants and seven HLA-mismatched transplants. The time interval from diagnosis to transplantation varied from 1 to 88 months, with a median of 3 months. Finally, patients received a median of 3.47×108/kg (0.85~11.20×108/kg) mononuclear cells (MNC). Eleven patients had positive serum HBsAg and three patients had positive serum HCV antibody tests before transplant. The total numbers of red blood cell and platelet transfusions were available for 49 patients, whereas information pertaining to the occurrence of transfusions, but not the total number, was available for the remaining. The median number of red blood cell and platelet transfusions before transplantation in these 49 patients was 5 (range 1~25) and 7 (range 1~40), respectively. All patients were followed for a median of 39 months (range 8 days to 194 months).
Engraftment
Myeloid engraftment occurred between 8 and 27 days (median 15 days) and platelet engraftment between 8 and 77 days (median 18 days). Three patients had primary graft failure (UPN 5, 22 and 302) and they died at 2.2, 4 and 3 months, respectively, after transplantation. Three patients (UPN 37, 73 and 89) rejected their bone marrow at 6, 7and 88 months, respectively, after transplantation, though the marrow was initially engrafted. UPN 37 died 1 week later because of sepsis. UPN 73 experienced marrow recovery after full dose CSA, prednisolone and azathioprine. See above under Patient characteristics for UPN 89.
GVHD
Fourteen of 73 evaluable patients (19.2%) had acute GVHD. Grade I acute GVHD occurred in nine patients (12.3%), grade II in three (4.1%), grade III in one (1.4%) and grade IV in one (1.4%). The grade I or II acute GVHD was controlled by intensification of immunosuppression. However, patients with grade III (UPN 76) and grade IV (UPN 52) GVHD died 2 and 1 month after transplantation, respectively. Twenty-three (34.8%) of 66 evaluable patients experienced chronic GVHD, 17 (25.8%) with limited and six (9.1%) with extensive stage disease. Age at transplant, gender, donor stem cell source (sibling vs. non-sibling), HLA compatibility (matched vs. mismatched), mononuclear cell dose, presence of HBsAg before transplant, interval from diagnosis to transplant, CMV disease, and number of red blood cell and platelet transfusions before transplant were analyzed for GVHD risk. Risk factors for acute GVHD included non-sibling stem cell source (P=0.004) and donor recipient mismatch (p=0.035). The only factor influencing the occurrence of chronic GVHD was age more than 30 years (P=0.026).
Morbidity and mortality
Veno-occlusive disease was noted in three patients and idiopathic pneumonitis in one patient. CMV infection or reactivation (defined as positive serum CMV IgM after stem cell transplant, positive blood CMV culture, positive urine CMV culture, or positive urine CMV-polymerase chain reaction) was found in eight (10.5%) of 76 evaluable patients. Nevertheless, only two patients had CMV disease clinically (one patient died of CMV pneumonitis and the other recovered from CMV colitis). During the follow up, 19 patients (23.8%) died. The major cause of death was sepsis in five patients. Other causes included primary graft failure in three patients, secondary graft failure in one, pneumonia in four, acute respiratory distress syndrome (ARDS) in two, intracranial hemorrhage in one, fulminant hepatitis in one, viral encephalitis in one, and interstitial pneumonitis in one.
Survival analysis
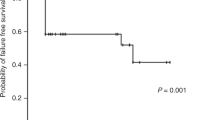
The projected 3- and 5-year overall survival estimated by the Kaplan-Meier method was 76.08 and 74.13%, respectively. Age at transplantation greater than 30 years (P=0.0131), stem cells from non-sibling donors (P=0.0356, Fig. 1), mononuclear cell dose greater than 3×108/kg (P=0.0472), grade II–IV acute GVHD (P=0.0000), time lag from diagnosis to transplantation longer than 12 months (P=0.0089), more than five red blood cell transfusions before transplantation (P=0.0318), and more than ten platelet transfusions before transplantation (P=0.0118) were poor prognostic factors by univariate analysis. Grade II–IV acute GVHD was the only significant prognostic factor after multivariate Cox regression analysis (P=0.04) (Table 2). Although patients who received transplant after 1992 had better survival than those who had transplant before 1992, the difference was not significant (P=0.2529).
Discussion
HSCT is an effective therapy for SAA patients, especially for younger patients who have an HLA-identical sibling donor [4, 6, 30]. Patients may also receive HSC from HLA-mismatched sibling donors [21, 42], HLA-matched unrelated donors [2, 3, 16, 18, 19] or the patient’s parents [40]. Reports of two large studies were published recently. On the basis of data from the International Bone Marrow Transplant Registry, the 5-year overall survival rate of 1,699 patients who received HLA-identical sibling transplants between 1991 and 1997 was around 70% [7]. Bacigalupo and colleagues from the European Group for Blood and Marrow Transplantation (EBMT) estimated the 5-year survival of 1,757 patients with SAA after 1990 was 80% [2]. These reports show that the survival rate has greatly improved since the 1970s. The probability of 3-year survival in patients with SAA who received HSCT in our hospital is 76.08%, which is comparable with the rates in these recent reports. If we measure the survival of recipients of sibling donor transplants exclusively, the rate approaches 80%.
Graft failure due to rejection or other causes is an important complication following allogeneic bone marrow transplantation for SAA [7, 15]. The incidence ranges from 5 to 50%, averaging about 10% [2, 17]. Some transplant strategies have been employed to reduce graft failure, including efforts to increase the donor cell dose transfused [23, 24, 25, 35, 36], to intensify pre-transplant conditioning [25], and to intensify post-transplant immunosuppression by CSA [7, 15, 22], with varying results. The major reduction in graft failure rate appeared in the early 1980s when CSA was used for post-transplant immunosuppression [15, 32]. Gluckman and her colleagues used a combination of cyclophosphamide, limited field radiation, CSA and MTX as treatment regimen for patients with SAA. Their reported graft failure rate was 10% [15]. Although addition of radiation to the conditioning regimen could effectively decrease graft failure rate to less than 5% [7, 12, 15, 27], no survival benefit was found because of radiation-associated complications [2, 15]. We used total body radiation plus cyclophosphamide as a conditioning regimen, and CSA plus MTX for post-transplant immunosuppression. The graft failure rate was 7.5% in our patients and was comparable to other recent reports.
Mortality of SAA patients with grade II–IV acute GVHD, especially with grades III or IV, is high. The risk of grade II–IV acute GVHD ranges from 15 to 20% in children and from 40 to 45% in older adults receiving transplants from HLA-identical sibling donors [17]. Immunosuppression by CSA plus MTX resulted in a lower incidence of acute GVHD than by either CSA or MTX alone [32, 33]. Storb and colleagues demonstrated that 18% of patients given MTX plus CSA had grade II–IV acute GVHD compared with 53% of those given MTX alone [32]. Irradiation was not given to their patients. Gluckman et al. reported a 21% incidence of acute GVHD in patients given cyclophosphamide, CSA and MTX [15]. Of our 73 evaluable patients, only five (6.8%) had grade II–IV acute GVHD. Our previous reports also found a lower incidence of acute GVHD in patients with SAA [8, 41]. The incidence of acute GVHD in our patients seemed to be lower than that reported by authors from Western countries. Similarly, Au et al. from Hong Kong also found only one patient had grade I cutaneous acute GVHD in 12 SAA patients receiving CSA and MTX [1]. We suggest that the lower acute GVHD may be related to ethnicity.
Extensive chronic GVHD occurred in 10–50% of recipients of HLA-identical sibling transplants [2, 17, 33]. A total of 25 and 9% of our patients had limited and extensive chronic GVHD, respectively. Reported risk factors included prior acute GVHD, older age, infusion of donor buffy coat cells, and radiation in the conditioning regimen [17, 34]. We found age older than 30 years (p=0.026) was the only risk factor for chronic GVHD in our patients.
We used the Kaplan-Meier method for univariate analysis of overall survival. Age older than 30 years, non-sibling donor, mononuclear cell dose larger than 3×108/kg, acute GVHD, time interval from diagnosis of SAA to transplant longer than 1 year, more than five red cell transfusions before transplantation and more than ten platelet transfusions before transplantation were significant poor prognostic factors. Gender, chronic GVHD, and status of HBsAg before transplantation were not prognostic factors. However, grade II–IV acute GVHD was the sole prognostic factor identified by multivariate analysis using Cox regression model (p=0.04).
There are many concerns about the late effects of total body irradiation-containing regimens such as secondary malignancy [11], growth retardation [9, 10, 29] and infertility [5, 20, 28]. Deeg and his colleagues reported 23 secondary malignancies in 700 patients with SAA treated with allogeneic marrow transplantation [11]. There were two acute lymphoblastic leukemia, three lymphoproliferative disorders and 18 solid tumors. At our institute, there have been no secondary malignancies in 71 patients who survived at least 2 months after transplantation. Regarding growth retardation, 26 of our patients received stem cell transplantation before or at the onset of puberty, with a median age of 10.0 years (range 4–15 years). Twelve of them reached their final height and two were considered a subnormal height (excess two standard deviation scores for the mean of the normal population). Although growth retardation had been attributed to total body irradiation [9], sometimes it was thought to be transient and not severe [10, 29]. Cohen and his colleagues stated that, despite a decrease in height standard deviation score values found after BMT, the majority of patients (140/181) had reached adult height that was within the normal range of the general population [10]. Regarding infertility, high-dose chemotherapy and total body irradiation have been implicated in ovarian failure and uterine dysfunction [5, 20, 28]. All of our female patients experienced gonadal failure and amenorrhea within 6 months after transplantation. Although four of our patients have married, none of them has had a pregnancy yet.
In conclusion, SAA patients can be managed by HSC transplantation with a good long-term outcome in Taiwanese. The major obstacles are GVHD and infection. Efforts must therefore be directed at prevention and management of acute GVHD and reduction of infection. The lower incidence of acute GVHD in our patients also warrants further investigation.
References
Au WY, Lie AK, Kwong YL, Chan TK, Chim CS, Lee CK, Chiu EK, Liang R (1998) Allogeneic bone marrow transplantation for severe aplastic anemia: the Hong Kong scenario. Hematol Oncol 16:41–46
Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J, Locasciulli A, Lint MTV, Tichelli A, McCann S, Marsh J, Ljungman P, Hows J, Marin P, Schrezenmeier H (2000) Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy—the European group for blood and marrow transplantation experience. Semin Hematol 37:69–80
Ball SE (2000) The modern management of severe aplastic anemia. Br J Haematol 110:41–53
Bortin MM, Gale RP, Rimm AA (1981) Allogeneic bone marrow transplantation for 144 patients with severe aplastic anemia. JAMA 245:1132–1139
Brennan BM, Shalet SM (2002) Endocrine late effects after bone marrow transplant. Br J Haematol 118:58–66
Camitta BM, Storb R, Thomas ED (1982) Aplastic anemia: pathogenesis, diagnosis, treatment and prognosis. N Engl J Med 306:645–652
Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, Gluckman E, Good RA, Rimm AA, Rozman C, Speck B, Bortin MM (1989) Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood 73:606–613
Chen PM, Tzeng CH, Fan FS, Hsieh RK, Wei CH (1994) Bone marrow transplantation in Taiwan: low incidence of acute GVHD in patients with hematologic malignancies and severe aplastic anemia. Bone Marrow Transplant 13:709–711
Clement-De Boers A, Oostdijk W, Van Weel-Sipman MH, Van den Broeck J, Wit JM, Vossen JM (1996) Final height and hormonal function after bone marrow transplantation in children. J Pediatr 129:544–550
Cohen A, Rovelli A, Bakker B, Uderzo C, van Lint MT, Esperou H, Gaiero A, Leiper AD, Dopfer R, Cahn JY, Merlo F, Kolb HJ, Socie G (1999) Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: a study by the Working Party for Late Effects-EBMT. Blood 93:4109–4115
Deeg HJ, Socie G, Schoch G, Henry-Amar M, Witherspoon RP, Devergie A, Sullivan KM, Gluckman E, Storb R (1996) Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood 87:386–392
Feig SA, Champlin R, Arenson E, Yale C, Ho W, Tesler A, Gale RP (1983) Improved survival following bone marrow transplantation for aplastic anemia. Br J Haematol 54:509–517
Gale RP, Ho W, Feig S, Champlin R, Tesler A, Arenson E, Ladish S, Young L, Winston D, Sparkes R, Fitchen J, Territo M, Sarna G, Wong L, Paik Y, Bryson Y, Golde D, Fahey J, Cline M (1981) Prevention of graft rejection following bone marrow transplantation. Blood 57:9–12
Gaziev D, Giardini C, Galimberti M, Lucarelli G, Angelucci E, Polchi P, Baronciani D, Erer B, Sotti G (1999) Bone marrow transplantation for transfused patients with severe aplastic anemia using cyclophosphamide and total lymphoid irradiation as conditioning therapy: long-term follow-up from a single center. Bone Marrow Transplant 24:253–257
Gluckman E, Horowitz MM, Champlin RE, Hows JM, Bacigalupo A, Biggs JC, Camitta BM, Gale RP, Gordon-Smith EC, Marmont AM, Masaoka T, Ramsay NKC, Rimm AA, Rozman C, Sobocinski KA, Speck B, Bortin MM (1992) Bone marrow transplantation for severe aplastic anemia: influence of conditioning and graft-versus-host disease prophylaxis regimens on outcome. Blood 79:269–275
Gordon-Smith EC, Fairhead SM, Chipping PM, Hows J, James DC, Dodi A, Batchelor JR (1982) Bone marrow transplantation for severe aplastic anemia using histocompatible unrelated volunteer donors. Br Med J 285:835–837
Horowitz MM (2000) Current status of allogeneic bone marrow transplantation in acquired aplastic anemia. Semin Hematol 37:30–42
Hows JM, Yin JL, Marsh J, Swirsky D, Jones L, Apperley JF, Fames DC, Smithers S, Batchelor JR, Goldman JM (1986) Histocompatible unrelated volunteer donors compared with HLA nonidentical family donors in marrow transplantation for aplastic anemia and leukemia. Blood 68:1322–1328
Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin R, Filipovich A, Gajewski J, Hansen JA, Henslee-Downey J, McCullough J (1993) Analysis of 462 transplantations from unrelated donors facilitated by The National Marrow Donor Program. N Engl J Med 328:593–602
Littley MD, Shalet SM, Morgenstern GR, Deakin DP (1991) Endocrine and reproductive dysfunction following fractionated total body irradiation in adults. Q J Med 78:265–274
Locatelli F, Porta F, Zecca M, Pedrazzoli P, Maccario R, Giani S, Vitale V, Martinetti M, Mazzolari E, Lanfranchi A (1993) Successful bone marrow transplantation in children with severe aplastic anemia using HLA-partially matched family donors. Am J Hematolol 42:328–333
McCann SR, Bacigalupo A, Gluckman E, Hinterberger W, Hows J, Ljungman P, Marin P, Nissen C, van’t Veer Kerthof E, Raghavachar A (1994) Graft rejection and second bone marrow transplants for acquired aplastic anemia: a report from the Aplastic Anaemia Working Party of the European Bone Marrow Transplant Group. Bone Marrow Transplant 13:233–237
Min CK, Kim DW, Lee JW, Han CW, Min WS, Kim CC (2001) Hematopoietic stem cell transplantation for high-risk adult patients with severe aplastic anemia; reduction of graft failure by enhancing stem cell dose. Haematologica 86:303–310
Niederwieser D, Pepe M, Storb R, Loughran TP Jr, Longton G (1988) Improvement in rejection, engraftment rate and survival without increase in graft-versus-host disease by high marrow cell dose in patients transplanted for aplastic anemia. Br J Haematol 69:23–28
Passweg JR, Socie G, Hinterberger W, Bacigalupo A, Biggs JC, Camitta BM, Champlin RE, Gale RP, Gluckman E, Gordon-Smith EC, Hows JM, Klein JP, Nugent ML, Pasquini R, Rowlings PA, Speck B, Tichelli A, Zhang MJ, Horowitz MM, Bortin MM (1997) Bone marrow transplantation for severe aplastic anemia: Has outcome improved? Blood 90:858–864
Paulin T (1992) Importance of bone marrow cell dose in bone marrow transplantation. Clin Transplant 6:48–54
Ramsay NK, Kim TH, McGlave P, Goldman A, Nesbit ME Jr, Krivit W, Woods WG, Kersey JH (1983) Total lymphoid irradiation and cyclophosphamide conditioning prior to bone marrow transplantation for patients with severe aplastic anemia. Blood 62:622–626
Sanders JE, Buckner CD, Amos D, Levy W, Appelbaum FR, Doney K, Storb R, Sullivan KM, Witherspoon RP, Thomas ED (1988) Ovarian function following marrow transplantation for aplastic anemia or leukemia. J Clin Oncol 6:813–818
Shinohara O, Kato S, Yabe H, Yabe M, Kubota C, Mitsuda R, Kimura M (1991) Growth after bone marrow transplantation in children. Am J Pediatr Hematol Oncol 13:263–268
Speck B (1991) Allogeneic bone marrow transplantation for severe aplastic anemia. Semin Hematol 28:319–321
Storb R (1993) Bone marrow transplantation for aplastic anemia. Cell Transplant 2:365–379
Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Hansen J (1986) Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood 68:119–125
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V, Hansen J, Hill R, Lum L, Martin P, McGuffin R, Sanders J, Stewart P, Sullivan K, Witherspoon R, Rharm GY, Thomas ED (1986) Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 314:729–735
Storb R, Prentice RL, Sullivan KM, Shulman HM, Deeg HJ, Doney KC, Buckner CD, Clift RA, Witherspoon RP, Appelbaum FR, Sanders JE, Stewart PS, Thomas ED (1983) Predictive factors in chronic graft-versus-host disease in patients with aplastic anemia treated by marrow transplantation from HLA-identical siblings. Ann Intern Med 98:461–466
Storb R, Prentice RL, Thomas ED (1977) Marrow transplantation of aplastic anemia: an analysis of factors associated with graft rejection. N Engl J Med 296:61–66
Storb R, Prentice RL, Thomas ED, Appelbaum FR, Deeg HJ, Doney K, Fefer A, Goodell BW, Mickelson E, Stewart P (1983) Factors associated with graft rejection after HLA-identical marrow transplantation for aplastic anemia. Br J Haematol 55:573–585
Storb R, Thomas ED, Buckner CD, Clift RA, Johnson FL, Fefer A, Glucksberg H, Giblett ER, Lerner KG, Neiman P (1974) Allogeneic marrow grafting for treatment of aplastic anemia. Blood 43:157–180
Thomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE, Lerner KG, Glucksberg H, Buckner CD (1975) Bone marrow transplantation. New Engl J Med 292:832–843, 895–902
Thomas ED, Storb R, Fefer A, Slichter SJ, Bryant JI, Buckner CD, Neiman PE, Clift RA, Funk DD, Lerner KE (1972) Aplastic anemia treated by marrow transplantation. Lancet 1:284–289
Tzeng CH, Chen PM, Fan S, Liu JH, Chiou TJ, Hsieh RK (1996) CT/TBI-800 as a pretransplant regimen for allogeneic bone marrow transplantation for severe aplastic anemia using HLA-haploidentical family donors. Bone Marrow Transplant 18:273–277
Tzeng CH, Hsieh RK, Fan S, Liu JH, Liu JM, Liu CJ, Chen KY, Yung CH, Wang SY, Wang SR, Chiou TJ, Chen PM (1992) Bone marrow transplantation for severe aplastic anemia—a study of twenty-one Chinese patients in Taiwan. Transplantation 53:569–574
Wagner JL, Deeg HJ, Seidel K, Anasetti C, Doney K, Sanders J, Sullivan KM, Storb R (1996) Bone marrow transplantation for severe aplastic anemia from genotypically HLA-nonidentical relatives. An update of the Seattle experience. Transplantation 61:54–61
Acknowledgements
This work was supported in part by a grant from the Yen Tjing-Lin Medical Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, L.Y., Chiou, T.J., Liu, J.H. et al. Hematopoietic stem cell transplantation for severe aplastic anemia—experience of an institute in Taiwan. Ann Hematol 83, 38–43 (2004). https://doi.org/10.1007/s00277-003-0781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-003-0781-3