Abstract
Purpose
The purpose of the study is to report 12-month efficacy and safety results from the subgroup of Japanese patients in the prospective IMPERIAL 2:1 randomized controlled trial (RCT).
Methods
The global IMPERIAL RCT was designed to compare performance of the Eluvia Drug-Eluting Vascular Stent System (Boston Scientific, Marlborough, MA, USA) with the Zilver PTX Drug-Eluting Peripheral Stent (Cook Medical, Bloomington, IN, USA) for treatment of femoropopliteal artery lesions. Patients with symptomatic (Rutherford category 2–4) disease were included. Post-procedural technical success was defined as delivery and deployment of the assigned study stent to the target lesion to achieve residual angiographic stenosis no greater than 30% by visual assessment. Twelve-month assessments included primary patency (core laboratory-assessed duplex ultrasound peak systolic velocity ratio ≤ 2.4 in the absence of clinically driven TLR or bypass of the target lesion) and major adverse events (MAEs).
Results
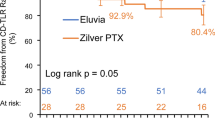
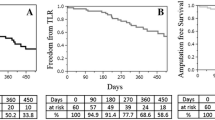
Fifty-six patients in the Eluvia group and 28 in the Zilver PTX group were treated at Japanese centers. Mean lesion length was 91.8 ± 38.0 mm for Eluvia and 87.4 ± 41.7 mm for Zilver PTX. Technical success was 100% for both groups. At 12 months, the observed primary patency rate was 90.9% for Eluvia and 84.6% for Zilver PTX. The 12-month MAE rate was 1.8% for Eluvia and 7.7% for Zilver PTX. All MAEs were clinically driven TLRs.
Conclusion
The results show excellent vessel patency and a good safety profile up to 1 year in the subgroup of Japanese patients in IMPERIAL treated with Eluvia for femoropopliteal artery disease.
Level of Evidence
Level 3; subgroup analysis of randomized trial.
Clinical Trial Registration
ClinicalTrials.gov, identifier NCT02574481.

Similar content being viewed by others
References
Gray WA, Keirse K, Soga Y, et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet. 2018;392:1541–51.
Müller-Hülsbeck S, Keirse K, Zeller T, Schroe H, Diaz-Cartelle J. Twelve month results from the MAJESTIC trial of the Eluvia paclitaxel-eluting stent for treatment of obstructive femoropopliteal disease. J Endovasc Ther. 2016;23:701–7.
Müller-Hülsbeck S, Keirse K, Zeller T, Schroe H, Diaz-Cartelle J. Long-term results from the MAJESTIC trial of the Eluvia paclitaxel-eluting stent for femoropopliteal treatment: 3-year follow-up. Cardiovasc Intervent Radiol. 2017;40:1832–8.
Bisdas T, Beropoulis E, Argyriou A, Torsello G, Stavroulakis K. 1-Year all-comers analysis of the Eluvia drug-eluting stent for long femoropopliteal lesions after suboptimal angioplasty. JACC Cardiovasc Interv. 2018;11:957–66.
Dake MD, Van Alstine WG, Zhou Q, Ragheb AO. Polymer-free paclitaxel-coated Zilver PTX stents—evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011;22:603–10.
Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016;133:1472–83.
Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504.
Ohki T, Yokoi H, Kichikawa K, et al. Two-year analysis of the Japanese cohort from the Zilver PTX randomized controlled trial supports the validity of multinational clinical trials. J Endovasc Ther. 2014;21:644–53.
Yokoi H, Ohki T, Kichikawa K, et al. Zilver PTX post-market surveillance study of paclitaxel-eluting stents for treating femoropopliteal artery disease in Japan: 12-month results. JACC Cardiovasc Interv. 2016;9:271–7.
Iida O, Takahara M, Soga Y, et al. One-year results of the ZEPHYR (Zilver PTX for the femoral artery and proximal popliteal artery) registry: predictors of restenosis. JACC Cardiovasc Interv. 2015;8:1105–12.
Soga Y, Inoue K, Kuma S. Pathological findings of late stent thrombosis after paclitaxel-eluting stent implantation for superficial femoral artery disease. J Cardiol Cases. 2015;11:39–41.
Müller-Hülsbeck S. Eluvia peripheral stent system for the treatment of peripheral lesions above the knee. Expert Opin Drug Deliv. 2016;13:1639–44.
Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–9.
Kichikawa K, Ichihashi S, Yokoi H, et al. Zilver PTX post-market surveillance study of paclitaxel-eluting stents for treating femoropopliteal artery disease in Japan: 2-year results. Cardiovasc Intervent Radiol. 2019;42:358–64.
Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e011245.
August 7, 2019 UPDATE: treatment of peripheral arterial disease with paclitaxel-coated balloons and paclitaxel-eluting stents potentially associated with increased mortality. U.S. Food and Drug Administration; 2019 (updated August 7, 2019; cited 2019 September 9, 2019). https://www.fda.gov/medical-devices/letters-health-care-providers/august-7-2019-update-treatment-peripheral-arterial-disease-paclitaxel-coated-balloons-and-paclitaxel.
Stone GW, Ellis SG, Colombo A, et al. Long-term safety and efficacy of paclitaxel-eluting stents final 5-year analysis from the TAXUS clinical trial program. JACC Cardiovasc Interv. 2011;4:530–42.
Acknowledgements
The authors thank the following Boston Scientific employees for their assistance: H. Terry Liao, PhD designed the statistical analysis plan, Naoko Takahashi and Rieko Kuribayashi provided clinical trial management, and Elizabeth J. Davis, PhD provided medical writing assistance.
Funding
This study was sponsored by Boston Scientific, Marlborough, MA, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yoshimitsu Soga serves as an advisor to Boston Scientific. Masahiko Fujihara, Osamu Iida, Daizo Kawasaki, Keisuke Hirano, Hiroyoshi Yokoi, Akira Miyamoto, Kimihiko Kichikawa, Masato Nakamura and Takao Ohki reports consulting for Boston Scientific. Juan Diaz-Cartelle is an employee of and owns stock in Boston Scientific. William A. Gray serves as an advisor to Boston Scientific. Stefan Müller-Hülsbeck serves as a consultant and has received honoraria and travel grants from Boston Scientific and has received fees from Terumo.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board, Independent Ethics Committee, or Research Ethics Board applicable to each study site, and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soga, Y., Fujihara, M., Iida, O. et al. Japanese Patients Treated in the IMPERIAL Randomized Trial Comparing Eluvia and Zilver PTX Stents. Cardiovasc Intervent Radiol 43, 215–222 (2020). https://doi.org/10.1007/s00270-019-02355-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02355-x