Abstract
Introduction
To determine whether post-treatment magnetic resonance imaging (MRI)-based texture analysis of liver metastases (LM) may be suited predicting therapy response to transarterial radioembolization (TARE) during follow-up.
Materials and Methods
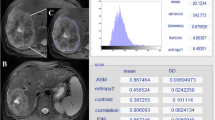
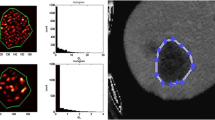
Thirty-seven patients with LM treated by TARE (mean age 63.4 years) between January 2006 and December 2014 were identified in this retrospective feasibility study. They underwent dynamic contrast-enhanced and hepatocellular phase MRI after TARE (mean 2.2 days). Response was evaluated on follow-up imaging scheduled in intervals of 3 months (median follow-up, 7.3 months) based on response evaluation criteria in solid tumors 1.1 (RECIST 1.1). Results of texture analysis [mean, standard deviation, skewness (s), kurtosis (k), entropy and uniformity] were compared between patients with progressive disease (PD) and patients with stable disease (SD), partial or complete response (PR/CR). Receiver operating characteristics including the area under the curve (AUC) and cutoff values including the sensitivity and specificity were calculated.
Results
According to RECIST 1.1, 24 patients (64.9%) had PD, 8 SD (21.6%) and 5 PR (13.5%). MRI-based texture analysis showed an earlier differentiation between patients with and without PD when compared with RECIST 1.1. Median k (2.88 vs. 2.35) in arterial phase MRI and median s (0.48 vs. 0.25) and k (2.85 vs. 2.25) in venous phase MRI were significantly different (p < 0.05). The AUC for k derived from arterial phase MRI was 0.73 (cutoff = 2.55, sensitivity = 0.83, specificity = 0.62) (p < 0.05). The AUC for s and k in venous phase MRI was 0.76 (cutoff = 0.35, sensitivity = 0.71, specificity = 0.85) (p > 0.05) and 0.83 (cutoff = 2.50, sensitivity = 0.75, specificity = 0.85) (p < 0.05).
Conclusion
This study indicates the potential of MRI-based texture analysis at arterial and venous phase MRI for the early prediction of PD after TARE.
Level of Evidence
IV.






Similar content being viewed by others
References
Kasper HU, Drebber U, Dries V, Dienes HP. Liver metastases: incidence and histogenesis. Z Gastroenterol. 2005;43:1149–57.
Nachtnebel A, Sotti G, Vitale A, Perrini MR. Selective internal radiotherapy using yttrium-90 microspheres for primary and secondary liver malignancies. Vienna: Ludwig Boltzmann Institute for Health Technology Assessment (LBI-HTA). 2011. http://eprints.hta.lbg.ac.at/922/1/DSD_47_english.pdf. Accessed 08 May 2015.
Clark ME, Smith RR. Liver-directed therapies in metastatic colorectal cancer. J Gastrointest Oncol. 2014;5:374–87.
Mahnken AH. Current status of transarterial radioembolization. World J Radiol. 2016;8:449–59.
Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507.
Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–78.
Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol. 2012;23:96–105.
Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol. 2008;19:1187–95.
Memon K, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83:887–94.
Saxena A, Chua TC, Bester L, Kokandi A, Morris DL. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251:910–6.
Gonzalez-Guindalini FD, Botelho MP, Harmath CB, et al. Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics. 2013;33:1781–800.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Singh P, Anil G. Yttrium-90 radioembolization of liver tumors: what do the images tell us? Cancer Imaging. 2013;13:645–57.
Davnall F, Yip CSP, Ljungvist G, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–89.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images Are More than Pictures, they are data. Radiology. 2016;278:563–77.
Alic L, Niessen WJ, Veenland JF. Quantification of heterogeneity as a biomarker in tumor imaging: a systematic review. PLoS ONE. 2014;9:e110300.
Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014;111(12):2205–13.
Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer Imaging. 2013;13:140–9.
Reiner CS, Gordic S, Puippe G, et al. Histogram analysis of CT perfusion of hepatocellular carcinoma for predicting response to transarterial radioembolization: value of tumor heterogeneity assessment. Cardiovasc Interv Radiol. 2016;39:400–8.
Morsbach F, Pfammatter T, Reiner CS, et al. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial radioembolization of liver metastases. Invest Radiol. 2013;48:787–94.
Morsbach F, Sah BR, Spring L, et al. Perfusion CT best predicts outcome after radioembolization of liver metastases: a comparison of radionuclide and CT imaging techniques. Eur Radiol. 2014;24:1455–65.
Reiner CS, Morsbach F, Sah BR, et al. Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vasc Interv Radiol. 2014;25:747–59.
Garin E, Lenoir L, Rolland Y, et al. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: preliminary results. J Nucl Med. 2012;53:255–63.
Zhu X, Sobhani F, Xu C, Pan L, Ghasebeh MA, Kamel IR. Quantitative volumetric functional MR imaging: an imaging biomarker of early treatment response in hypo-vascular liver metastasis patients after yttrium-90 transarterial radioembolization. Abdom Radiol (NY). 2016;41:1495–504.
Schmeel FC, Simon B, Luetkens JA, et al. Prognostic value of pretreatment diffusion-weighted magnetic resonance imaging for outcome prediction of colorectal cancer liver metastases undergoing 90Y-microsphere radioembolization. J Cancer Res Clin Oncol. 2017;143:1531–41.
Kokabi N, Camacho JC, Xing M, et al. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39:969–78.
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725–48.
Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–78.
Mahnken AH, Spreafico C, Maleux G, Helmberger T, Jakobs TF. Standards of practice in transarterial radioembolization. Cardiovasc Interv Radiol. 2013;36:613–22.
Lewandowski RJ, Sato KT, Atassi B, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Interv Radiol. 2007;30:571–92.
Padia SA, Lewandowski RJ, Johnson GE, et al. Radioembolization of hepatic malignancies: background, quality improvement guidelines, and future directions. J Vasc Interv Radiol. 2017;28:1–15.
Mann HB, Whitney DR. On a test of whether one of 2 random variables is stochastically larger than the other. Annals Math Stat. 1947;18:50–60.
Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristic curves. Crit Care. 2004;8:508–12.
Yang X, Knopp MV. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol. 2011;2011:732848.
Simpson-Herren L, Noker PE, Wagoner SD. Variability of tumor response to chemotherapy. II. Contribution of tumor heterogeneity. Cancer Chemother Pharmacol. 1988;22:131–6.
Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–57.
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol. 2012;22:796–802.
De Cecco CN, Ganeshan B, Ciolina M, et al. Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal Cancer patients studied with 3-T magnetic resonance. Invest Radiol. 2015;50(4):239–45.
Chen BB, Shih TT. DCE-MRI in hepatocellular carcinoma-clinical and therapeutic image biomarker. World J Gastroenterol. 2014;20:3125–34.
Chandarana H, Rosenkrantz AB, Mussi TC, et al. Histogram analysis of whole-lesion enhancement in differentiating clear cell from papillary subtype of renal cell cancer. Radiology. 2012;265:790–8.
Downey K, Riches SF, Morgan VA, et al. Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. AJR Am J Roentgenol. 2013;200:314–20.
O’Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21:249–57.
Chapiro J, Duran R, Lin M, et al. Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver. Eur Radiol. 2015;25:1993–2003.
Tacher V, Lin M, Duran R, et al. Comparison of existing response Criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization Using a 3D quantitative approach. Radiology. 2016;278:275–84.
Acknowledgements
The authors thank Prof. Dr. Nina Timmesfeld (Philipps-University, Marburg, Germany) for statistical advice, Dr. Matthias Baumhauer (Mint Medical GmbH, Dossenheim, Germany) for technical support and Dr. Andrew McIntyre (Städtisches Klinikum Karlsruhe, Germany) for language editing. Our special thanks go to the liver tumor board at Klinikum Karlsruhe and the interventional section of the Institute of Diagnostic and Interventional Radiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
The requirement to obtain an additional informed consent was waived by the ethics committee of Philipps-University, Marburg, Germany.
Rights and permissions
About this article
Cite this article
Reimer, R.P., Reimer, P. & Mahnken, A.H. Assessment of Therapy Response to Transarterial Radioembolization for Liver Metastases by Means of Post-treatment MRI-Based Texture Analysis. Cardiovasc Intervent Radiol 41, 1545–1556 (2018). https://doi.org/10.1007/s00270-018-2004-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-2004-2