Abstract
Purpose
To evaluate in vivo the role of RAGE (receptor for advanced glycated end products) in the development of restenosis and neointimal proliferation in RAGE-deficient knockout (KO) mice compared with wild-type (WT) mice in an animal model.
Materials and Methods
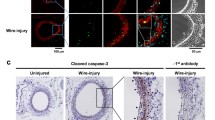
Sixteen WT and 15 RAGE-deficient mice underwent microvascular denudation of the common femoral artery under general anaesthesia. Contralateral arteries underwent a sham operation and served as controls. Four weeks after the intervention, all animals were killed, and paraformaldehyde-fixed specimens of the femoral artery were analysed with different stains (hematoxylin and eosin and Elastica van Gieson) and several different types of immunostaining (proliferating cell nuclear antigen, α-actin, collagen, von Willebrand factor, RAGE). Luminal area, area of the neointima, and area of the media were measured in all specimens. In addition, colony-formation assays were performed, and collagen production by WT smooth muscle cells (SMCs) and RAGE-KO SMCs was determined. For statistical analysis, P < 0.05 was considered statistically significant.
Results
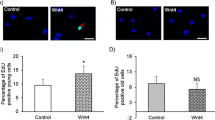
Four weeks after denudation, WT mice showed a 49.6% loss of luminal area compared with 14.9% loss of luminal area in RAGE-deficient mice (sham = 0% loss) (P < 0.001). The neointima was 18.2 (*1000 μm2 [n = 15) in the WT group compared with only 8.4 (*1000 μm2 [n = 16]) in the RAGE-KO group. RAGE-KO SMCs showed significantly decreased proliferation activity and production of extracellular matrix protein.
Conclusion
RAGE may be shown to play a considerable role in the formation of neointima leading to restenosis after vascular injury.





Similar content being viewed by others
References
Schleicher E, Friess U (2007) Oxidative stress, AGE, and atherosclerosis. Kidney Int 2007 106(1):S17–S26
Duda SH, Bosiers M, Lammer J et al (2006) Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther 13(6):701–710
Ritthaler U, Deng Y, Zhang Y et al (1995) Expression of receptors for advanced glycation end products in peripheral occlusive vascular disease. Am J Pathol 146(3):688–694
Sakaguchi T, Yan SF, Yan SD et al (2003) Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest 111(7):959–972
Yamagishi S, Matsui T, Nakamura K (2007) Kinetics, role and therapeutic implications of endogenous soluble form of receptor for advanced glycation end products (sRAGE) in diabetes. Curr Drug Targets 8(10):1138–1143
Ihara Y, Egashira K, Nakano K et al (2007) Upregulation of the ligand-RAGE pathway via the angiotensin II type I receptor is essential in the pathogenesis of diabetic atherosclerosis. J Mol Cell Cardiol 43(4):455–464
Wang L, Li S, Jungalwala FB (2008) Receptor for advanced glycation end products (RAGE) mediates neuronal differentiation and neurite outgrowth. J Neurosci Res 86(6):1254–1266
Tesarova P, Kalousova M, Jachymova M et al (2007) Receptor for advanced glycation end products (RAGE)–soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Invest 25(8):720–725
Constien R, Forde A, Liliensiek B et al (2001) Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 30(1):36–44
Feuls R, Chereshnev I, Bantleon R et al (2003) Microvascular denudation of the femoral artery of the mouse as a model for restenosis. Rofo 175(7):952–957
Kobayashi M, Inoue K, Warabi E et al (2005) A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb 12(3):138–142
Jansson G (1991) Oestrogen-induced enhancement of myeloperoxidase activity in human polymorphonuclear leukocytes—a possible cause of oxidative stress in inflammatory cells. Free Radic Res Commun 14(3):195–208
Miyata T, Ishikawa S, Asahi K et al (1999) 2-Isopropylidenehydrazono-4-oxo-thiazolidin-5-ylacetanilide (OPB-9195) treatment inhibits the development of intimal thickening after balloon injury of rat carotid artery: role of glycoxidation and lipoxidation reactions in vascular tissue damage. FEBS Lett 445(1):202–206
Anderson MM, Requena JR, Crowley JR et al (1999) The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest 104(1):103–113
Li J, Qu X, Schmidt AM (1998) Sp1-binding elements in the promoter of RAGE are essential for amphoterin-mediated gene expression in cultured neuroblastoma cells. J Biol Chem 273(47):30870–30878
Li J, Schmidt AM (1997) Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem 272(26):16498–16506
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418(6894):191–195
Schmidt AM, Stern D (2000) Atherosclerosis and diabetes: the RAGE connection. Curr Atheroscler Rep 2(5):430–436
Lander HM, Tauras JM, Ogiste JS et al (1997) Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem 272(28):17810–17814
Bierhaus A, Schiekofer S, Schwaninger M et al (2001) Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 50(12):2792–2808
Bierhaus A, Nawroth PP (2009) Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 52(11):2251–2263
Oldfield MD, Bach LA, Forbes JM et al (2001) Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest 108(12):1853–1863
Throckmorton DC, Brogden AP, Min B et al (1995) PDGF and TGF-beta mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int 48(1):111–117
Farb A, Kolodgie FD, Hwang JY et al (2004) Extracellular matrix changes in stented human coronary arteries. Circulation 110(8):940–947
Ikonomidis JS, Jones JA, Barbour JR et al (2007) Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg 133(4):1028–1036
Baker AH, Edwards DR, Murphy G (2002) Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115(Pt 19):3719–3727
Hofmann Bowman M, Wilk J, Heydemann A et al (2010) S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res 106(1):145–154
Kamioka M, Ishibashi T, Ohkawara H et al (2011) Involvement of membrane type 1-matrix metalloproteinase (MT1-MMP) in RAGE activation signaling pathways. J Cell Physiol 226(6):1554–1563
Schober W, Wiskirchen J, Kehlbach R et al (2002) Antiproliferative effects of the antiallergic agent azelastine on human aortic smooth-muscle cells: an in vitro study. Invest Radiol 37(5):248–253
Kubota Y, Kichikawa K, Uchida H et al (1995) Pharmacologic treatment of intimal hyperplasia after metallic stent placement in the peripheral arteries. An experimental study. Invest Radiol 30(9):532–537
Tepe G, Brehme U, Seeger H et al (2002) Endothelin A receptor antagonist LU 135252 inhibits hypercholesterolemia-induced, but not deendothelialization-induced, atherosclerosis in rabbit arteries. Invest Radiol 37(6):349–355
Watson AM, Soro-Paavonen A, Sheehy K et al (2011) Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia 54:681–689
Yamagishi S, Nakamura K, Matsui T et al (2008) Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: a novel therapeutic strategy for diabetic vascular complications. Expert Opin Invest Drugs 17(7):983–996
Takagi T, Yamamuro A, Tamita K et al (2003) Pioglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with type 2 diabetes mellitus: an intravascular ultrasound scanning study. Am Heart J 146(2):E5
Choi D, Kim SK, Choi SH et al (2004) Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care 27(11):2654–2660
Barile GR, Schmidt AM (2007) RAGE and its ligands in retinal disease. Curr Mol Med 7(8):758–765
van Zoelen MA, Yang H, Florquin S et al (2009) Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31(3):280–284
Acknowledgments
This study was supported in part by grants from the interdisciplinary center for clinical research, University of Tübingen. This publication reflects only the authors’ views. The authors are not liable for any use that may be made of information herein.
Conflict of interest
Gerd Grözinger has no conflict of interest to declare. Jörg Schmehl has no conflict of interest to declare. Rüdiger Bantleon has no conflict of interest to declare. Rainer Kehlbach has no conflict of interest to declare. Tarun Mehra has no conflict of interest to declare. Claus D. Claussen has no conflict of interest to declare. Benjamin Wiesinger has no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grözinger, G., Schmehl, J., Bantleon, R. et al. Decreased Neointimal Extracellular Matrix Formation in RAGE-Knockout Mice After Microvascular Denudation. Cardiovasc Intervent Radiol 35, 1439–1447 (2012). https://doi.org/10.1007/s00270-011-0319-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-011-0319-3