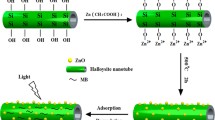
Abstract
Co3O4 nanoparticles were successfully deposited on the surface of natural halloysite nanotubes (HNTs) to produce Co3O4/HNTs composites. The structure and morphology of the samples were characterized using X-ray diffraction, field-emission scanning electron microscope, transmission electron microscope and Fourier transform infrared. The results indicated that Co3O4 nanoparticles were uniformly attached on the surface of HNTs with narrow size distribution. Co3O4/HNTs exhibited an excellent photocatalytic efficiency for degradation of methyl blue under UV light, better than Co3O4 and HNTs mixture, HNTs and pure Co3O4. The mechanism of enhanced photocatalytic activity of Co3O4/HNTs was also proposed.











Similar content being viewed by others
References
Abdullayev E, Price R, Shchukin D, Lvov Y (2009) Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole. ACS Appl Mater Interfaces 1:1437–1443
Artero V, Chavarot-Kerlidou M, Fontecave M (2011) Splitting water with cobalt. Angew Chem Int Ed 50:7238–7266
Azadi P, Farnood R, Meier E (2010) Preparation of multiwalled carbon nanotube-supported nickel catalysts using incipient wetness method. J Phys Chem A 114:3962–3968
Cao AM, Hu JS, Liang HP, Song WG, Wan LJ, He XL, Gao XG, Xia SH (2006) Hierarchically structured cobalt oxide (Co3O4) the morphology control and its potential in sensors. J Phys Chem B 110:15858–15863
Casas-Cabanas M, Binotto G, Larcher D, Lecup A, Giordani V, Tarascon J (2009) Defect chemistry and catalytic activity of nanosized Co3O4. Chem Mater 174:1939–1947
Chen L, Yin S-F, Huang R, Zhang Q, Luo SL, Au CT (2012) Hollow peanut-like m-BiVO4: facile synthesis and solar-light-induced photocatalytic property. CrystEngComm 14:4217–4222
Delannoy L, El Hassan N, Musi A, Le To NN, Krafft J-M, Louis C (2006) Preparation of supported gold nanoparticles by a modified incipient wetness impregnation method. J Phys Chem B 110:22471–22478
Du ML, Guo BC, Jia DM (2010) Newly emerging applications of halloysite nanotubes: a review. Polym Int 59:574–582
Endo M, Strano MS, Ajayan PM (2008) Potential applications of carbon nanotubes. Carbon Nanotubes 111:13–62
Gualtieri AF (2001) Synthesis of sodium zeolites from a natural halloysite. Phys Chem Miner 28:719–728
Guimarães L, Enyashin AN, Seifert G, Duarte HA (2010) Structural, electronic, and mechanical properties of single-walled halloysite nanotube models. J Phys Chem C 114:11358–11363
Hu PW, Yang HM (2012) Sb-SnO2 nanoparticles onto kaolinite rods: assembling process and interfacial investigation. Phys Chem Miner 39:339–349
Hu LH, Peng Q, Li YD (2008) Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J Am Chem Soc 130:16136–16137
Hughes AD, King MR (2010) Use of naturally occurring halloysite nanotubes for enhanced capture of flowing cells. Langmuir 26:12155–12164
Jiao F, Frei H (2009) Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew Chem Int Ed 48:1841–1844
Joussein E, Pettit S, Churchman J, Theng B, Righi D, Delvaux B (2005) Halloysite clay minerals a review. Clay Miner 40:383–426
Levis SR, Deasy PB (2002) Characterisation of halloysite for use as a microtubular drug delivery system. Int J Pharm 243:125–134
Li W, Jung H, Hoa ND, Kim D, Hong S-K, Kim H (2010) Nanocomposite of cobalt oxide nanocrystals and single-walled carbon nanotubes for a gas sensor application. Sens Actuators B Chem 150:160–166
Liang YY, Li YG, Wang HL, Zhou JG, Wang J, Regier T, Dai HJ (2011) Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater 10:780–786
Liu B, Aydil ES (2009) Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J Am Chem Soc 131:3985–3990
Lvov YM, Shchukin DG, Möhwald H, Price RR (2008) Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2:814–820
Natile MM, Glisenti A (2002) Study of surface reactivity of cobalt oxides interaction with methanol. Langmuir 14:3090–3099
Pauporté T, Mendoza L, Cassir M, Bernard MC, Chivot J (2005) Direct low-temperature deposition of crystallized CoOOH films by potentiostatic electrolysis. J Electrochem Soc 152:C49–C53
Shchukin DG, Lamaka SV, Yasakau KA, Zheludkevich ML, Ferreira MGS, Mohwald H (2008) Active anticorrosion coatings with halloysite nanocontainers. J Phys Chem C 112:958–964
Takada S, Fujii M, Kohiki S (2001) Intraparticle magnetic properties of Co3O4 nanocrystals. Nano Lett 1:379–382
Veerabadran NG, Mongayt D, Torchilin V, Price RR, Lvov YM (2009) Organized shells on clay nanotubes for controlled release of macromolecules. Macromol Rapid Commun 30:99–103
Wang L, Chen J, Ge L, Zhu Z, Rudolph V (2011) Halloysite-nanotube-supported ru nanoparticles for ammonia catalytic decomposition to produce COx-free hydrogen. Energy Fuels 25:3408–3416
Woan K, Pyrgiotakis G, Sigmund W (2009) Photocatalytic carbon-nanotube-TiO2 composites. Adv Mater 21:2233–2239
Wöllenstein J, Burgmair M, Plescher G, Sulima T, Hildenbrand J, Böttner H, Eisele I (2003) Cobalt oxide based gas sensors on silicon substrate for operation at low temperatures. Sens Actuators B Chem 93:442–448
Yeo BS, Bell AT (2011) Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J Am Chem Soc 133:5587–5593
Zhai R, Zhang B, Liu L, Xie YD, Zhang HQ, Liu JD (2010) Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal Commun 12:259–263
Zhang Y, Yang HM (2012) Halloysite nanotubes coated with magnetic nanoparticles. Appl Clay Sci 56:97–102
Acknowledgments
This work was supported by the National Natural Science Foundation of China (50774095) and the Scientific Research Foundation for ROCS of SEM (2011-1139).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Yang, H. Co3O4 nanoparticles on the surface of halloysite nanotubes. Phys Chem Minerals 39, 789–795 (2012). https://doi.org/10.1007/s00269-012-0533-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-012-0533-9