Abstract
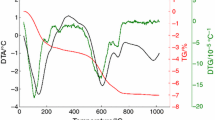
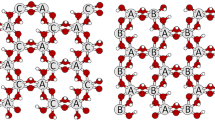
Illite is a dioctahedral K-deficient mica with an interlayer cation content of 0.6–0.85 atoms per formula unit. 1M and 2M1 are the illite polytypes more abundant in nature. Because illite is one of the major component of clays used for the production of traditional ceramics, the understanding of its high temperature transformations is of paramount importance for the knowledge of the structural and microstructural properties of fired ceramic products. To our knowledge, the study of the illite dehydroxylation kinetics has not been attempted to date. Hence, this work presents the investigation of the reaction mechanism of dehydroxylation of illite for the first time. The natural sample investigated in this study is a 1M-polytype from Hungary. Several classical methods of kinetic analysis were used (isoconversional method, Avrami method, direct fit with kinetic expressions, and others) to achieve a complete picture of the dehydroxylation mechanism. The proposed model for the dehydroxylation of illite is a multi-step reaction sequence with (1) condensation of the water molecule in the octahedral layer; (2) one-dimensional diffusion of the water molecules through the tetrahedral ring (rate limiting step of the reaction); (3) two-dimensional diffusion of the water molecules through the interlayer region (rate limiting step of the reaction).










Similar content being viewed by others
References
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11(7):36–42
Arletti R (2005) The ancient Roman glass: an archaeometrical investigation. PhD Thesis, University of Modena and Reggio Emilia, Modena
Baitalow F, Schmidt HG, Wolf G (1999) Formal kinetic analysis of processes in the solid state. Thermo Acta 337:111–120
Bamford CH, Tipper CHF (1980) Comprehensive chemical kinetics, vol 22. Reactions in the solid state. Elsevier, Amsterdam
Bellotto M, Gualtieri AF, Artioli G, Clark SM (1995) Kinetic study of the kaolinite-mullite reaction sequence. I. Kaolinite dehydroxylation. Phys Chem Miner 22:207–214
Bose K, Ganguly J (1994) Thermogravimetric study of the dehydration kinetics of talc. Am Mineral 79:692–699
Bray HJ, Redfern AT (2000) Influence of counterion species on the dehydroxylation of Ca2+-, Mg2+-, Na+- and K+-exchanged Wyoming montmorillonite. Min Mag 64(2):337–346
Brigatti MF, Guggenheim S (2002) Mica crystal chemistry and the influence of pressure, temperature, and solid solution on atomistic models. Mineral Soc Am Rev Mineral 46:1–97
Brindley GW (1975) Thermal transformations of clays and layer silicates. In: Proceedings of the international clay conference, Mexico City, Mexico. Applied Publishing, Wilmette, pp 119–129
Cruciani G, Gualtieri AF (1999) Dehydration dynamics of analcime using synchrotron powder diffraction. Am Mineral 84:112–119
Drits VA, Besson G, Muller F (1995) An improved model for structural transformations of heat-treated aluminous dioctahedral 2:1 layer silicates. Clays Clay Miner 43:718–731
Eberl DD, Drits VA, Środoń J, Nüesch R (1996) MudMaster: a computer program for calculating crystallite size distributions and strain from the shapes of X-ray diffraction peaks, U.S. Geological Survey Open File Report 96.171, 44 pp
Ferrari S, Gualtieri AF, Grathoff GH, Leoni M (2006) Model of structure disorder of illite: preliminary results. Zeit für Krist, Supplement No. 23. In: Proceedings of European Powder Diffraction Conference (EPDIC 9) (in press)
Földvári M (1997) Kaolinite-genetic and thermoanalytical parameters. J Therm An 48:107–119
Friedman HL (1964) Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C Polym Lett 6:183–195
Grim RE, Bray RH, Bradley WF (1937) The mica in argillaceous sediments. Am Mineral 22:813–829
Guggenheim S, Chang YH, Van Groos AFK (1987) Muscovite dehydroxylation: high temperature studies. Am Mineral 72:537–550
Hancock JD, Sharp JH (1972) Method of comparing solid-state kinetic data and its application to the decomposition of kaolinite, brucite and BaCO3. J Am Cer Soc 55:74–77
Hulbert SF (1969) Models for solid state reaction in powdered compacts: a review. J Br Soc 6:11–20
Inoue A, Kohyama N, Kitagawa R, Watanabe T (1987) Chemical and morphological evidence for the conversion of smectite to illite. Clays Clay Miner 35:111–120
Jackson ML (1969) Soil chemical analyses—advanced course, 2nd edn. Published by the author, Department of Soil Science, University of Wisconsin, Madison
Kodama H, Brydon JE (1968) Dehydroxylation of microcrystalline muscovite. Trans Faraday Soc 63:3112–3119
Koga N, Criado JM (1998) Kinetic analyses of solid-state reactions with a particle-size distribution. J Am Ceram Soc 81(11):2901–2909
MacKenzie RC (1970) Differential thermal analysis, vol 1. Academic, London
Mazzuccato E, Artioli G, Gualtieri A (1999) High temperature dehydroxylation of muscovite−2M1: a kinetic study by in situ XRPD. Phys Chem Minerals 26:375–381
Muller F, Drits VA, Plançon A, Robert JL (2000) Structural transformation of 2:1 dioctahedral layer silicates during dehydroxylation–rehydroxylation reactions. Clays Clay Miner 48:572–585
Nicol A W (1964) Topotactic transformation of muscovite under mild hydrothermal conditions. Clays Clay Miner 12:11–19
Norrish K, Pickering JG (1983) Clay minerals. In: CSIRO (ed) Soils, an Australian viewpoint. Division of Soils, CSIRO. Academic, London, pp 281–308
Rasband WS (1997–2005) ImageJ, U. S. National Institutes of Health, Bethesda, http://www.rsb.info.nih.gov/ij/
Reynolds RC Jr (1985) NEWMOD: a copyrighted computer program for the calculation of basal X-ray diffraction intensities of mixed-layered clays. R.C. Reynolds, Hanover
Reynolds RC Jr (1993) Three-dimensional X-ray powder diffraction from disordered illite: simulation and interpretation of the diffraction patterns. In: Reynolds RC, Walker JR Jr (eds) Computer applications to X-ray powder diffraction analysis of clay minerals, vol 5. Clay Minerals Society, Boulder, pp 43–78
Reynolds RC Jr (1994) WILDFIRE: a copyrighted computer program for the calculation of three-dimensional powder X-ray diffraction patterns for mica polytypes and their disordered vacations. R.C. Reynolds, Hanover
Rieder M, Cavazzini G, D’Yakonov YS, Frank-Kamenetskii VA, Gottardi G, Guggenheim S, Koval’ PV, Müller G, Neiva AMR, Radoslovich EW, Robert JL, Sassi FP, Takeda H, Weiss Z, Wones DR (1998) Nomenclature of the micas. Clays Clay Miner 46:586–595
Rodriguez-Navarro C, Cultrone G, Sanchez-Navas A. Sebastian E (2003) TEM study of mullite growth after muscovite breakdown. Am Mineral 88:713–724
Rosenberg PE (2002) The nature, formation, and stability of end-member illite: a hypothesis. Am Mineral 87:103–107
Rouxhet PG (1970) Kinetics of dehydroxylation and of OH–OD exchange in macrocrystalline micas. Am Mineral 55:841–853
Sanchez-Navas A (1999) Sequential kinetics of a muscovite-out reaction: a natural example. Am Mineral 84:1270–1286
Sainz-Díaz CI, Escamilla-Roa E, Hernández-Laguna A (2004) Pyrophyllite dehydroxylation process by first-principles calculations. Am Mineral 89:1092–1100
Środoń J, Eberl DD (1984) Illite. Mineral Soc Am Rev Mineral 13:495–544
Svedberg T, Nichols JB (1923) Determination of size and distribution of size of particle by centrifugal methods. J Am Chem Soc 45:2910–2917
Wang L, Zhang M, Redfern SAT, Zhang Z (2002) Dehydroxylation and transformations of the 2:1 Phyllosilicate pyrophyllite at elevated temperatures: an infrared spectroscopic study. Clays Clay Min 50(2):272–283
Webster CE, Drago RS, Zerner MC (1998) Molecular dimensions for adsorptives. J Am Chem Soc 120:5509–5516
Wenk HR, Bulakh A (2004) Minerals. Cambridge University Press, Cambridge
Yates DM, Rosenberg PE (1996) Formation and stability of end-member illite: I. Solution equilibration experiments at 100–250°C and Pv,soln. Geoch Cosmo Acta 60:1873–1883
Yates DM, Rosenberg PE (1997) Formation and stability of end-member illite: II. Solid equilibration experiments at 100 to 250°C and Pv,soln. Geoch Cosmo Acta 61:3135–3144
Acknowledgments
This work is part of the PhD thesis of S. F. Thanks to A. Csebi and J. Biber for letting us inside the Füzérradvány mine to collect the illite specimen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gualtieri, A.F., Ferrari, S. Kinetics of illite dehydroxylation. Phys Chem Minerals 33, 490–501 (2006). https://doi.org/10.1007/s00269-006-0092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-006-0092-z