Abstract
Background/objective
Quick optimization and mastery of a new technique is an important part of procedural medicine, especially in the field of minimally invasive surgery. Complex surgeries such as robotic pancreaticoduodenectomies (RPD) and robotic distal pancreatectomies (RDP) have a steep learning curve; therefore, findings that can help expedite the burdensome learning process are extremely beneficial. This single-surgeon study aims to report the learning curves of RDP, RPD, and robotic Heller myotomy (RHM) and to review the results’ implications for the current state of robotic hepatopancreaticobiliary (HPB) surgery.
Study design
This is a retrospective case series of a prospectively maintained database at a non-university tertiary care center. Total of 175 patients underwent either RDP, RPD, or RHM with the surgeon (DRJ) from January 2014 to January 2020.
Results
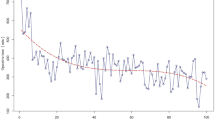
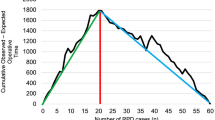
Statistical significance of operating room time (ORT) was noted after 47 cases for RDP (p < 0.05), 51 cases for RPD (p < 0.0001), and 18 cases for RHM (p < 0.05). Mean ORT after the statistical mastery of the procedure for RDP, RPD, and RHM was 124, 232, 93 min, respectively. No statistical significance was noted for estimated blood loss or length of stay.
Conclusions
Robotic HPB procedures have significantly higher learning curves compared to non-HPB procedures, even for an experienced HPB surgeon with extensive laparoscopic experience. Our RPD curve, however, is quicker than the literature average. We suggest that this is because of the simultaneous implementation of HPB (RDP and RPD) and non-HPB robotic surgeries with a shorter learning curve—especially foregut procedures such as RHM—into an experienced surgeon’s practice. This may accelerate the learning process without compromising patient safety and outcomes.



Similar content being viewed by others
References
Strijker MCV, Santvoort HG, Besselink MV et al (2013) Robot-assisted pancreatic surgery: a systematic review of the literature. Hpb 15(1):1–10. https://doi.org/10.1111/j.1477-2574.2012.00589.x
Giulianotti PC, Mangano A, Bustos RE et al (2018) Operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique. Surg Endosc 32:4329–4336. https://doi.org/10.1007/s00464-018-6228-7
Orti-Rodríguez RJ, Rahman SH (2014) A comparative review between laparoscopic and robotic pancreaticoduodenectomies. Surg Laparosc, Endosc and Percutaneous Tech 24(2):103–108. https://doi.org/10.1097/sle.0b013e3182a2f0ad
Subramonian K, Muir G (2014) The learning curve in surgery: what is it, how do we measure it and can we influence it? BJU Int 93(9):1173–1174. https://doi.org/10.1111/j.1464-410x.2004.04891.x
Lim TO, Soraya A, Ding LM et al (2002) Assessing doctors’ competence: application of CUSUM technique in monitoring doctors’ performance. Int J Qual Health Care 14(3):251–258. https://doi.org/10.1093/oxfordjournals.intqhc.a002616
Wohl H (1977) The cusum plot: its utility in the analysis of clinical data. N Engl J Med 296(18):1044–1045. https://doi.org/10.1056/nejm197705052961806
Cho E, Pagkratis S, Osman H et al (2020) Robotic pancreatoduodenectomy. minimally invasive surgical techniques for cancers of the gastrointestinal tract. A Step-by-Step Approach Chapter 13:p123-132. https://doi.org/10.1007/978-3-030-18740-8_13
Shakir M, Boone BA, Polanco PM et al (2015) The learning curve for robotic distal pancreatectomy: an analysis of outcomes of the first 100 consecutive cases at a high-volume pancreatic centre. Hpb 17(7):580–586. https://doi.org/10.1111/hpb.12412
Yamaguchi T, Kinugasa Y, Shiomi A et al (2014) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29(7):1679–1685. https://doi.org/10.1007/s00464-014-3855-5
Napoli N, Kauffmann EF, Perrone VG et al (2015) The learning curve in robotic distal pancreatectomy. Updates Surg 67:257–264. https://doi.org/10.1007/s13304-015-0299-y
Boone BA, Zenati M, Hogg ME et al (2015) Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 150(5):416–422. https://doi.org/10.1001/jamasurg.2015.17
Shi Y, Jin J, Qiu W et al (2020) Short-term outcomes after robot-assisted vs open pancreaticoduodenectomy after the learning curve. JAMA Surg 155(5):372–455. https://doi.org/10.1001/jamasurg.2020.0021
Shyr B-U, Chen S-C, Shyr Y-M et al (2018) Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Medicine 97(45):e13000. https://doi.org/10.1097/md.0000000000013000
Enayati N, Momi ED, Ferrigno G (2016) Haptics in robot-assisted surgery: challenges and benefits. IEEE Rev Biomed Eng 9:49–65. https://doi.org/10.1109/rbme.2016.2538080
Ross SB, Kurian TJ, Luberice K et al (2011) Defining the learning curve of laparo-endoscopic single site (less) heller myotomy. Gastroenterology 140(5):72–77. https://doi.org/10.1016/s0016-5085(11)64295-9
Yano F, Omura N, Tsuboi K et al (2017) Learning curve for laparoscopic Heller myotomy and Dor fundoplication for achalasia. Plos One 12(7):e0180515. https://doi.org/10.1371/journal.pone.0180515
Jackson T, Cho EE, Nagatomo K et al (2020) Teacher and trainee learning together-dual console and the 3 arms. J Surg Edu S1931–7204(20):30013–30021. https://doi.org/10.1016/j.jsurg.2020.01.013
van der Schans EM, Hiep MAJ, Consten ECJ et al (2020) From Da Vinci Si to Da Vinci Xi: realistic times in draping and docking the robot. J Robotic Surg. https://doi.org/10.1007/s11701-020-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, J.S., Jackson, T., Kurtz, J. et al. Overcoming the Arduous Transition for Robotic Hepatopancreatobiliary Cases: A Multi-Procedure Learning Curve Study Utilizing CUSUM Analysis. World J Surg 45, 865–872 (2021). https://doi.org/10.1007/s00268-020-05861-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05861-z