Abstract
Background
Sarcopenia at time of diagnosis predicts worse survival outcomes. It is currently unknown how changes in muscle mass over time interact with sarcopenia in colorectal patients treated with curative intent. Objectives of this study were to quantify sarcopenia and skeletal muscle loss from time of diagnosis to end of surveillance and determine its effect on survival outcomes after completion of 2 years of surveillance.
Methods
Retrospective cohort study of stage I–III colorectal cancer patients from 2007–2009, who underwent resection and had preoperative and 2-year surveillance computed tomography scans, without recurrence during that time. Body composition analysis was done at both time points to determine lumbar skeletal muscle index, radiodensity and adiposity. Change over time was standardized as a percentage per year. Cox proportional hazard regression modeling was used for survival analysis.
Results
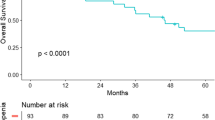
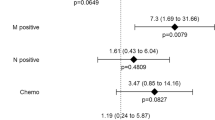
Of 667 patients included, median survival from surgery was 7.96 years, with 75 recurrences occurring after 2 years. On average patients lost muscle mass (−0.415%/year; CI −0.789, −0.042) and radiodensity (−5.76 HU/year; CI −6.74, −4.80), but gained total adipose tissue (7.06%/year; CI 4.34, 9.79). Patients with sarcopenia at diagnosis (HR 1.80; CI 1.13, 2.85) or muscle loss over time (HR 1.55; CI 1.01, 2.37) had worse overall survival, with significantly worse joint effect (HR 2.73; CI 1.32, 5.65).
Conclusions
Sarcopenia at diagnosis combined with ongoing skeletal muscle loss over time resulted in significantly worse survival. Patients with these features who are recurrence-free at 2 years are more likely to have a non-colorectal cancer cause of death.

Similar content being viewed by others
References
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Malietzis G, Currie AC, Athanasiou T et al (2016) Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg 103:572–580
Prado CMM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9:629–635
McSorley ST, Black DH, Horgan PG et al (2017) The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr 37(4):1279–1285
Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA et al (2017) Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol 3:e172319
Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA et al (2016) Metabolic dysfunction, obesity, and survival among patients with early-stage colorectal cancer. J Clin Oncol 34:3664–3671
Caan BJ, Meyerhardt JA, Kroenke CH et al (2017) Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS study). Cancer Epidemiol Biomarkers Prev 26:1008–1015
Malietzis G, Aziz O, Bagnall NM et al (2015) The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol 41:186–196
Huang DD, Wang SL, Zhuang CL et al (2015) Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis 17:O256–264
Nakanishi R, Oki E, Sasaki S et al (2018) Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today 48(2):151–157
Prado CM, Birdsell LA, Baracos VE (2009) The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 3:269–275
Margadant CC, Bruns ER, Sloothaak DA et al (2016) Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur J Surg Oncol 42:1654–1659
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, et al (2016) Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 34:1339–1344
Malietzis G, Currie AC, Johns N et al (2016) Skeletal muscle changes after elective colorectal cancer resection: a longitudinal study. Ann Surg Oncol 23:2539–2547
Rutten IJ, van Dijk DP, Kruitwagen RF et al (2016) Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle 7:458–466
Deng CY, Lin YC, Wu JS et al (2018) Progressive sarcopenia in patients with colorectal cancer predicts survival. AJR Am J Roentgenol 210:526–532
Reisinger KW, Bosmans JW, Uittenbogaart M et al (2015) Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol 22:4445–4452
van der Werf A, Dekker IM, Meijerink MR et al (2018) Skeletal muscle analyses: agreement between non-contrast and contrast CT scan measurements of skeletal muscle area and mean muscle attenuation. Clin Physiol Funct Imaging 38(3):366–372
von Elm E, Altman D, Egger M et al (2007) The strengthening and reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Shen W, Punyanitya M, Wang Z et al (1985) (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97:2333–2338
Popuri K, Cobzas D, Esfandiari N et al (2016) Body composition assessment in axial CT images using fem-based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging 35:512–520
Harrell FE, Lee KL, Mark DB (1996) Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Williams BA, Mandrekar JN, Mandrekar SJ et al (2006) Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes: technical report. Division of Biostatistics, Mayo Clinic
Mourtzakis M, Prado CM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Vickers A, Altman D (2001) Analysing controlled trials with baseline and follow up measurements. BMJ 323:1123–1124
Vickers A (2001) The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 1:6
Meyerhardt JA, Kroenke CH, Prado CM et al (2017) Association of weight change after colorectal cancer diagnosis and outcomes in the Kaiser Permanente northern California population. Cancer Epidemiol Biomarkers Prev 26:30–37
Meyerhardt JA, Niedzwiecki D, Hollis D et al (2008) Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol 26:4109–4115
Campbell PT, Newton CC, Dehal AN et al (2012) Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 30:42–52
Baade PD, Meng X, Youl PH et al (2011) The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev 20:1410–1420
Abellan van Kan G (2009) Epidemiology and consequences of sarcopenia. J Nutr Health Aging 13:708–712
Moon SW, Choi JS, Lee SH et al (2019) Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res 20:35
Moisey L, Mourtzakis M, Cotton B et al (2013) Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Respir Res 17(5):R206
Montano-Loza AJ, Meza-Junco J, Prado CM et al (2012) Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 10:166–173
Rier HN, Jager A, Sleijfer S et al (2018) Changes in body composition and muscle attenuation during taxane-based chemotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat 168(1):95–105
Cooper AB, Slack R, Fogelman D et al (2015) Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 22:2416–2423
Daly LE, Ni Bhuachalla EB, Power DG et al (2018) Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle 9:315–325
Miyamoto Y, Baba Y, Sakamoto Y et al (2015) Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One [Electronic Resource] 10:e0129742
Di Sebastiano KM, Yang L, Zbuk K et al (2013) Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr 109:302–312
Dalal S, Hui D, Bidaut L et al (2012) Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manag 44:181–191
Lieffers JR, Mourtzakis M, Hall KD et al (2009) A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr 89:1173–1179
Acknowledgements
The authors acknowledge Arsene Zongo for his assistance with the optimal stratification analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hopkins, J.J., Reif, R., Bigam, D. et al. Change in Skeletal Muscle Following Resection of Stage I–III Colorectal Cancer is Predictive of Poor Survival: A Cohort Study. World J Surg 43, 2518–2526 (2019). https://doi.org/10.1007/s00268-019-05054-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05054-3