Abstract
Background
Critical limb ischemia (CLI) caused by peripheral arterial disease is associated with significant morbidity and mortality. This condition is associated with a 30 % amputation rate as well as mortality levels which might be as high as 25 %. There is no pharmacological therapy available, but several reports have suggested that mesenchymal stem cells (MSCs) may be a useful therapeutic option.
Methods
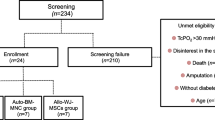
This study, done at a university hospital, evaluated 13 patients for a phase I trial to investigate the safety and efficacy of intra-arterial MSCs in CLI patients. Eight patients with ten affected limbs were recruited for the study. As two patients (three limbs) died of ischemic cardiac events during the 6-month follow-up period, seven limbs were finally evaluated for the study.
Results
There was significant pain relief. Visual analog scale (VAS) scores decreased from 2.29 ± 0.29 to 0.5 ± 0.34 (p < 0.05), ankle brachial pressure index (ABPI) increased significantly from 0.56 ± 0.02 to 0.67 ± 0.021 (p < 0.01), and transcutaneous oxygen pressure (TcPO2) also increased significantly in the foot from 13.57 ± 3.63 to 38 ± 3.47. Similar improvement was seen in the leg as well as the thigh. There was 86 % limb salvage and six of seven ulcers showed complete or partial healing.
Conclusion
It was concluded that intra-arterial MSCs could be safely administered to patients with CLI and was associated with significant therapeutic benefits.



Similar content being viewed by others
References
Hirsch AT, Haskal ZJ, Hertzer NR et al (2006) Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113:e463–e654
Anon (1996) Critical limb ischaemia: management and outcome. Report of a National Survey: The Vascular Society of Great Britain and Ireland. Eur J Vasc Endovasc Surg 12:131–135
Watt SM, Athanassopoulos A, Harris AL et al (2010) Human endothelial stem/progenitor cells, angiogenic factors and vascular repair. J R Soc Interface 7(Suppl 6):S731–S751
El Oakley RM, Seow KK, Tang TPL et al (2002) Whole bone marrow transplantation induces angiogenesis following acute ischemia. Redox Rep 7(4):215–218
Iba O, Matsubara H, Nozawa Y et al (2002) Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation 106(15):2019–2025
Kalka C, Masuda H, Takahashi T et al (2000) Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 97(7):3422–3427
Murphy MP, Lawson JH, Rapp BM et al (2011) Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg 53:1565–1574
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Tse WT, Pendleton JD, Beyer WM et al (2003) Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75(3):389–397
Di Nicola M, Carlo-Stella C, Magni M et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10):3838–3843
U.S. Food and Drug Administration, CFR Code of Federal Regulations Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=640. Accessed 5 Feb 2011
Walter DH, Krankenberg H, Balzer JO et al (2011) Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv 4:26–37
Tateishi-Yuyama E, Matsubara H, Murohara T et al (2002) Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 360:427–435
Esato K, Hamano K, Li TS et al (2002) Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant 11:747–752
Miyamoto M, Yasutake M, Takano H et al (2004) Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transpl 13:429–437
Saigawa T, Kato K, Ozawa T et al (2004) Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J 68:1189–1193
Iafrati MD, Hallett JW, Geils G et al (2011) Early results and lessons learned from a multicenter, randomized, double-blind trial of bone marrow aspirate concentrate in critical limb ischemia. J Vasc Surg 54(6):1650–1658
Levine JE, Harris RE, Loberiza FR et al (2003) A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood 101(7):2476–2482
Van Tongeren RB, Hamming JF, Fibbe WE et al (2008) Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: a clinical trial in patients with advanced limb ischemia. J Cardiovas Surg 49:51–58
Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M et al (2011) Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transpl 20:1629–1639
Schiavetta A, Maione C, Botti C et al (2012) A phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the Naples and Pietra Ligure evaluation of stem cells study. Stem Cells Trans Med 1:572–578
Acknowledgments
We acknowledge Stempeutics Research Malaysia (SRM) and University of Malaya (UM) for funding this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, A.K., Abdullah, B.J.J.B., Dhillon, S.S. et al. Intra-arterial Allogeneic Mesenchymal Stem Cells for Critical Limb Ischemia are Safe and Efficacious: Report of a Phase I Study. World J Surg 37, 915–922 (2013). https://doi.org/10.1007/s00268-012-1892-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1892-6