Abstract
Background
The purposes of the present study were to detect the expression of metastasis-associated protein 1 (MTA1) in patients with esophageal squamous cell cancer (ESCC), and to evaluate the relevance of MTA1 protein expression to the tumor progression, angiogenesis, and prognosis.
Methods
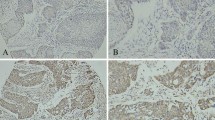
Both MTA1 protein and intratumoral microvessels were examined by immunohistochemical staining in 131 ESCC patients who successfully underwent subtotal esophagectomy and esophagogastric anastomosis at Qilu Hospital between Jan 2004 and Dec 2005. Intratumoral microvessel density (MVD) was recorded by counting CD-34 positive immunostained endothelial cells. All statistical analyses were performed with SPSS 13.0 statistical software.
Results
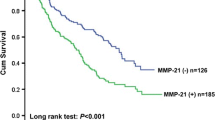
High expression of MTA1 protein was detected in 57 cases and significantly correlated with tumor invasion depth (P = 0.041), lymph node metastasis (P = 0.021), pathologic stage (P = 0.003), and MVD (P = 0.044). Survival analysis showed that patients with MTA1 protein high expression had significantly poor overall 5-year survival (P = 0.002), and the factor found on multivariate analysis to significantly affect overall survival was only pathologic stage (P = 0.040). Further stratified survival analysis split by pathologic stage demonstrated that MTA1 protein high expression significantly predicted unfavorable prognosis among patients with pathologic stage II disease (P = 0.006).
Conclusions
High expression of the MTA1 protein is common in ESCC, and is closely associated with tumor progression, increased tumor angiogenesis, and poor survival. These findings indicate that MTA1 protein has clinical potentials as a useful indicator of progressive phenotype, a promising prognostic predictor to identify patients with poor prognosis, and a potential novel therapeutic target of antiangiogenesis for patients with ESCC.




Similar content being viewed by others
References
Sano A, Kato H, Sakurai S et al (2009) CD24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol 16:506–514
Ren Y, Cao B, Law S et al (2005) Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res 11:6190–6197
Mariette C, Balon JM, Piessen G et al (2003) Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 97:1616–1623
Sugiura K, Ozawa S, Kitagawa Y et al (2007) Co-expression of aFGF and FGFR-1 is predictive of a poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep 17:557–564
Law S, Kwong DL, Kwok KF et al (2003) Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg 238:339–347
Okawa T, Naomoto Y, Nobuhisa T et al (2005) Heparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2. Clin Cancer Res 11:7995–8005
Shih CH, Ozawa S, Ando N et al (2000) Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 6:1161–1168
Toh Y, Nicolson GL (2009) The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clin Exp Metastasis 26:215–227
Toh Y, Pencil SD, Nicolson GL (1994) A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem 269:22958–22963
Nicolson GL, Nawa A, Toh Y et al (2003) Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer invasion, proliferation and nuclear regulation. Clin Exp Metastasis 20:19–24
Kumar R, Wang RA, Bagheri-Yarmand R (2003) Emerging roles of MTA family members in human cancers. Semin Oncol 30:30–37
Mazumdar A, Wang RA, Mishra SK et al (2001) Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol 3:30–37
Yan C, Wang H, Toh Y et al (2003) Repression of 92-kDa type IV collagenase expression by MTA1 is mediated through direct interactions with the promoter via a mechanism, which is both dependent on and independent of histone deacetylation. J Biol Chem 278:2309–2316
Jang KS, Paik SS, Chung H et al (2006) MTA1 overexpression correlates significantly with tumor grade and angiogenesis in human breast cancer. Cancer Sci 97:374–379
Martin MD, Hilsenbeck SG, Mohsin SK et al (2006) Breast tumors that overexpress nuclear metastasis-associated 1 (MTA1) protein have high recurrence risks but enhanced responses to systemic therapies. Breast Cancer Res Treat 95:7–12
Hofer MD, Kuefer R, Varambally S et al (2004) The role of metastasis-associated protein 1 in prostate cancer progression. Cancer Res 64:825–829
Balasenthil S, Broaddus RR, Kumar R (2006) Expression of metastasis-associate protein 1 (MTA1) in benign endometrium and endometrial adenocarcinomas. Hum Pathol 37:656–661
Ryu SH, Chung YH, Lee H et al (2008) Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology 47:929–936
Mahoney MG, Simpson A, Jost M et al (2002) Metastasis-associated protein (MTA) 1 enhances migration, invasion and anchorage-independent survival of immortalized human keratinocytes. Oncogene 21:2161–2170
Hofer MD, Menke A, Genze F et al (2004) Expression of MTA1 promotes motility and invasiveness of PANC-1 pancreatic carcinoma cells. Br J Cancer 90:455–462
Kidd M, Modlin IM, Mane SM et al (2006) The role of genetic markers—NAP1L1, MAGE-D2, and MTA1—in defining small-intestinal carcinoid neoplasia. Ann Surg Oncol 13:253–262
Vallböhmer D, Lenz HJ (2006) Predictive and prognostic molecular markers in outcome of esophageal cancer. Dis Esophagus 19:425–432
Shimada H, Matsushita K, Tagawa M (2008) Recent advances in esophageal cancer gene therapy. Ann Thorac Cardiovasc Surg 14:3–8
Moon HE, Cheon H, Chun KH et al (2006) Metastasis-associated protein 1 enhances angiogenesis by stabilization of HIF-1alpha. Oncol Rep 16:929–935
Kai L, Wang J, Ivanovic M et al (2011) Targeting prostate cancer angiogenesis through metastasis-associated protein 1 (MTA1). Prostate 71:268–280
Korst RJ, Rusch VW, Venkatraman E et al (1998) Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg 115:660–670
Hamilton SR, Aaltonen LA (2000) Tumours of the digestive system. In Pathology and Genetics, World Health Organization Classification of Tumours, IARC Press, Lyon, pp 10–25
Greene FL, Page DL, Fleming ID et al (2002) AJCC cancer staging manual, 6th edn. Springer, New York, pp 91–98
Qian H, Lu N, Xue L et al (2005) Reduced MTA1 expression by RNAi inhibits in vitro invasion and migration of esophageal squamous cell carcinoma cell line. Clin Exp Metastasis 22:653–662
Igarashi M, Dhar DK, Kubota H et al (1998) The prognostic significance of microvessel density and thymidine phosphorylase expression in squamous cell carcinoma of the esophagus. Cancer 82:1225–1232
Li SH, Wang Z, Liu XY (2009) Metastasis-associated protein 1 (MTA1) overexpression is closely associated with shorter disease-free interval after complete resection of histologically node-negative esophageal cancer. World J Surg 33:1876–1881
Situ DR, Hu Y, Zhu ZH et al (2010) Prognostic relevance of β-catenin expression in T2–3N0M0 esophageal squamous cell carcinoma. World J Gastroenterol 16:5195–5202
Djärv T, Lagergren P (2011) Six-month postoperative quality of life predicts long-term survival after oesophageal cancer surgery. Eur J Cancer 47:530–535
Nawa A, Nishimori K, Lin P et al (2000) Tumor metastasis-associated human MTA1 gene: its deduced protein sequence, localization, and association with breast cancer cell proliferation using antisense phosphorothioate oligonucleotides. J Cell Biochem 79:202–212
Toh Y, Ohga T, Endo K et al (2004) Expression of the metastasis-associated MTA1 protein and its relationship to deacetylation of the histone H4 in esophageal squamous cell carcinomas. Int J Cancer 110:362–367
Butler JM, Kobayashi H, Rafii S (2010) Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer 10:138–146
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Vermeulen PB, Gasparini G, Fox SB et al (2002) Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 38:1564–1579
Acknowledgments
The authors are grateful to Lutao Du (Department of Clinical Laboratory) for his data analysis from Qilu Hospital, Shandong University, China.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, SH., Tian, H., Yue, WM. et al. Metastasis-associated Protein 1 Nuclear Expression is Closely Associated with Tumor Progression and Angiogenesis in Patients with Esophageal Squamous Cell Cancer. World J Surg 36, 623–631 (2012). https://doi.org/10.1007/s00268-011-1421-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1421-z