Abstract
Objectives
This study was designed to evaluate Acinetobacter baumannii infections incidence in our Surgical Intensive Care Unit, clinical features and outcome of these patients, and multi-resistance incidence to identify predictors of such a resistance.
Methods
Prospective study of all patients with ICU-acquired Acinetobacter baumannii infection from June 1, 2003 to May 31, 2005. Patients with multi-resistant infection, susceptible exclusively to colistin, were compared with those sustaining non-multi-drug resistant infection.
Results
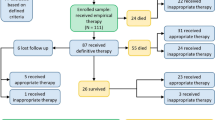
Among 411 patients, 52 (12.6%) developed Acinetobacter infection. Their mean age was 66.3 ± 8.4 years and APACHE II 20.4 ± 7.3 (men: 51.9%). Infection sites were: bloodstream (46.2%), respiratory tract (32.7%), central venous catheter (11.5%), surgical site (7.7%), and urinary tract (1.9%). High multi-resistance (44.2%), morbidity (63.4%), and mortality (44.2%) were identified. Colistin was the most effective antibiotic (100% susceptibility), whereas resistance against all other antibiotics was >60%. Previous septic shock (p = 0.04), previous adult respiratory distress syndrome (ARDS) (p = 0.01), number of previous antibiotics (p = 0.01), previous aminoglycoside use (p = 0.04), and reoperation (p = 0.01) were risk factors for multi-resistance in univariate analysis. Morbidity in the multi-resistant group was significantly higher than the non-multi-resistant group (82.6% vs. 48.2%, p = 0.02). Mortality in the multi-resistant group also was higher; however, this difference did not marginally reach statistical significance (60.8% vs. 31.1%, p = 0.06). Multivariate analysis identified previous septic shock (p = 0.04; odds ratio (OR), 9.83; 95% confidence interval (CI), 1.003–96.29) and reoperation (p = 0.01; OR, 8.45; 95% CI, 1.52–46.85) as independent predictors of multi-resistance.
Conclusion
Acinetobacter baumannii infections are frequent and associated with high morbidity, mortality, and multi-resistance. Avoidance of unnecessary antibiotics is a high priority, and specific attention should be paid to patients with previous ARDS and, particularly, previous septic shock and reoperation. When such risk factors are identified, colistin may be the only appropriate treatment.
Similar content being viewed by others
Introduction
Hospital infections are a common problem that is associated with significant morbidity and mortality along with rapidly increasing multi-resistance to antibiotics [1–6]. Intensive Care Unit (ICU) patients are at great risk of developing a hospital infection, whereas resistance rates of ICU-isolated pathogens to antimicrobial agents are substantially higher than those in community and hospital ward infections [1, 3, 4, 7]. High incidence of multi-resistant ICU infections is attributable to severe underlying disease and comorbidities, inappropriate use of broad-spectrum antibiotics, mechanical ventilation, utilization of devices and interventional techniques, and prolonged hospital stay [1, 3–12].
Although significant differences regarding occurrence rates of microorganisms causing ICU infections, infection sites incidence, and antimicrobial resistance profiles are identified in different countries and even ICUs in the same country [1–3, 6, 12], Acinetobacter baumannii has emerged as a common worldwide problem as a nosocomial pathogen in ICU patients that is associated with widespread resistance to the major groups of antibiotics and high infection-associated morbidity and mortality [2, 4–6, 8–16].
New antimicrobial agents with activity against Acinetobacter baumannii are not likely to be available in the near future, making ongoing surveillance of the activities of currently available agents very important. Moreover, studies that address potential risk factors would greatly improve our understanding of the epidemiology of antimicrobial resistance in institutions and guide efforts to develop more effective strategies for prevention and treatment. The objective of this study was to evaluate the incidence of Acinetobacter baumannii infections in our Surgical Intensive Care Unit (SICU) and the clinical features and outcome of these patients. Furthermore, we investigated the incidence of multi-resistant Acinetobacter baumannii and its antimicrobial susceptibility to identify risk factors and predictors of such a resistance.
Patients and methods
Study population
This prospective study was conducted in the Surgical Intensive Care Unit (SICU), 1st Department of Propaedeutic Surgery, Hippokrateion Hospital, University of Athens. All adult patients (older than aged 14 years) who developed an ICU-acquired infection by Acinetobacter baumannii, with clinical signs of infection and confirmed by culture, from June 1, 2003 to May 31, 2005 were included in the study. Colonizations, defined as any positive culture without clinical signs of infection, were excluded. Patients who did not sustain an infection also were excluded. In addition, all infections that occurred before patients’ admission to the SICU, within the first 48 h of SICU hospitalization, or after 48 h after their discharge were excluded. Finally, because there is no pediatric or pediatric surgical department in our hospital, patients younger than aged 14 years are not hospitalized in our unit. In conclusion, all adult patients who developed an ICU-acquired Acinetobacter baumannii infection in our SICU, with clinical signs of infection and confirmed by culture according to standard criteria of Centers for Disease Control and Prevention (CDC) [17, 18] and International Sepsis Forum (ISF) [19], regardless of infection site, from June 1, 2003 to May 31, 2005 were included in this prospective observational study. Institutional review board approval was obtained before study initiation.
Data collection and analysis
Registry data collection (including information before SICU admission, during SICU hospitalization until discharge or death, and after discharge from the SICU until discharge from the hospital) starts immediately after patients’ admission to the SICU and continues on a daily basis.
SICU patients with clinical signs of infection and a clinical specimen positive for Acinetobacter baumannii were identified and followed prospectively. Isolates were considered duplicates and excluded from the database if they were collected during any 7-day period from the same patient and had identical antibiograms. Identical isolates from different specimen sources collected within that same 7-day period also were excluded.
The isolates were identified by conventional methods. Antimicrobial resistance was determined according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) [20]. Intermediately susceptible isolates were regarded as resistant.
Cultures were performed based on the clinical picture of the patients. Patients with clinical signs of infection received empirical antimicrobial therapy. After culture results, all patients with documented infection were administered antibiotics according to standard sensitivity testing.
Recorded data in the study group patients were: age, sex, medical history, underlying surgical pathology (hospital admission diagnosis), APACHE II score [21] on the day of admission to the SICU, SOFA score [22] on the day of infection diagnosis, type of management (conservative or operative), time in hospital before SICU admission, antibiotics before infection (exposure, type, and duration), presence and duration of invasive procedures (arterial, central and peripheral venous lines, urinary and nasogastric/nasointestinal catheters, mechanical ventilatory support, enteral or parenteral nutrition, and renal replacement therapy), days of SICU hospitalization and postoperative days until Acinetobacter baumannii isolation, previous sepsis episodes, infection site, microbial susceptibility to antibiotics, concurrent infections, complications (such as organ/system failure, subsequent infections, peritonitis, and hemorrhage), length of total SICU and hospital stay, and final outcome.
Previous antibiotic exposure was defined as at least 48 h of therapy within the 14 days before Acinetobacter baumannii isolation. Organ/system failure was defined according to American College of Chest Physicians (ACCP) and Society of Critical Care Medicine (SCCM) criteria [23] as well as Society of Critical Care Medicine (SCCM)/European Society of Intensive Care Medicine (ESICM)/American College of Chest Physicians (ACCP)/American Thoracic Society (ATS)/Surgical Infection Society (SIS) criteria [24].
Multi-drug resistant infection was defined as an infection caused by Acinetobacter baumannii that was susceptible only to colistin, whereas non-multi-drug resistant as an infection susceptible to colistin and at least one more antibiotic. According to microbial susceptibility to antibiotics, patients were divided in two groups: those infected by multi-drug resistant Acinetobacter baumannii composed the multi-resistant group, and those with non-multi-drug resistant infection the non-multi-resistant group. To identify and evaluate potential predictive factors of multi-resistance, comparison between the two groups of patients was performed. Only variables occurring before infection were analyzed as possible predisposing factors.
Statistical analysis
Univariate analysis was conducted using Student’s t test for numeric variables and chi-square and Fisher’s exact test for categorical variables. Moreover, multivariate analysis was performed; a multivariate logistic regression model was created with multi-drug resistance as a dependent variable and all the other recorded variables with p < 0.2 in the univariate analysis as independent variables to identify independent predictors of multi-resistance. Data are presented as mean ± SEM (standard error of mean) or number of patients and percentage. Statistical significance was set to p < 0.05.
Results
Among 411 consecutive hospitalized SICU patients during the 2-year study period, 52 (12.6%) developed an Acinetobacter baumannii infection and formed our study group. Mean age of the patients was 66.3 ± 8.4 years and APACHE II score on the day of SICU admission was 20.4 ± 7.3. Mortality was 44.2% (n = 23). Demographic and clinical features along with the underlying surgical pathology of the total study group patients are presented in Table 1. On the day of infection caused by Acinetobacter baumannii, 45 (86.5%) of the 52 patients had fever (temperature > 38°C), 2 (3.9%) had hypothermia (temperature < 36°C), 21 (40.3%) had septic shock, 42 (80.8%) had tachycardia (heart rate > 90/min), 12 (23%) had tachypnea (respiratory rate > 20/min), 16 (30.7%) had acute respiratory failure, 14 (26.9%) had renal failure, 11 (21.1%) had heart failure, 7 (13.4%) had atrial fibrillation, 4 (7.7%) hepatic failure, 40 (77%) had white blood cell count > 12 x 109/l, 3 (5.7%) had white blood cell count < 4 x 109/l, 37 (71.1%) had metabolic acidosis, 10 (19.2%) had Paco2 < 32 mmHg, 4 (7.7%) had thrombocytopenia, and 2 (3.9%) had coagulopathy.
Comparison of the patients with Acinetobacter baumannii infection with those with infection caused by all other organisms revealed that Acinetobacter infection was associated with a significantly higher morbidity and mortality (63.4% vs. 43.9%, p = 0.03, and 44.2% vs. 26.3%, p = 0.04, respectively).
Our patients sustained 52 Acinetobacter baumannii infections. No patient developed a second infection by Acinetobacter baumannii. Infection sites were: blood (n = 24, 46.2%), respiratory tract (n = 17, 32.7%), central venous catheter (n = 6, 11.5%), surgical site (n = 4, 7.7%), and urinary tract (n = 1, 1.9%; Table 1). In two patients another organism was isolated along with Acinetobacter (pseudomonas aeruginosa in one and klebsiella pneumoniae in one case), whereas in the remaining patients Acinetobacter was isolated as a solitary organism.
Overall morbidity was 63.4% (n = 33). The most frequent complication was septic shock (n = 26, 50%) followed by respiratory failure (n = 23, 44.2%), subsequent infection (n = 21, 40.3%), and renal failure (n = 19, 36.6%). In addition, 16 patients (30.7%) developed thrombocytopenia, 15 (28.9%) had cardiac failure, 13 (25%) coagulopathy, 10 (19.2%) had liver failure, 3 (5.7%) had postoperative peritonitis, and 2 (3.9%) had postoperative hemorrhage.
Ten patients (19.2%) underwent reoperation: three patients with necrotic pancreatitis, three due to peritonitis caused by anastomotic leak, two because of hemorrhage, one patient with abdominal trauma and initial damage control surgery, and one with superior mesentery artery embolism (second-look operation).
Susceptibility to antimicrobial agents is presented in Table 2. Colistin was the most active antimicrobial agent (100% susceptibility), whereas resistance against all other antibiotics was > 60%. Multi-resistant Acinetobacter baumannii was identified in 23 patients (44.2%) who composed the multi-resistant group; the remaining 29 formed the non-multi-resistant group. Demographic and clinical data of the multi-resistant and the non-multi-resistant group as well as the results of the comparison between these two groups are shown in Table 1.
Univariate analysis for multi-resistant infection revealed a significant association with the number of antibiotics administered before Acinetobacter baumannii isolation (p = 0.01), previous aminoglycoside use (p = 0.04), previous adult respiratory distress syndrome (ARDS) or septic shock (p = 0.01 and p = 0.04, respectively), and reoperation (p = 0.01; Table 1). Moreover, as shown in the same table, morbidity of the patients with multi-resistant infections was significantly higher than those with non-multi-resistant (82.6% vs. 48.2%, p = 0.02). In addition, mortality in the multi-resistant group was higher than the non-multi-resistant group; however, this difference did not marginally reach statistical significance (60.8% vs. 31.1%, p = 0.06; Table 1).
Multivariate analysis showed that previous septic shock (p = 0.04; odds ratio (OR), 9.83; 95% confidence interval (CI), 1.003–96.29) and reoperation (p = 0.01; OR, 8.45; 95% CI, 1.52–46.85) are predictors of microbial multi-drug resistance (Table 3).
Discussion
The search for the means to understand, control, and prevent the emergence and spread of infections and antimicrobial resistance has become a public health priority [1, 3]. Infections in ICUs are a frequent, severe, and continuously increasing problem [1–6, 9, 12, 16, 25, 26]. Treatment of these infections is very challenging because of the rapidly progressing multi-resistance of the causing microorganisms to the existing antibiotics and the lack of effective new antimicrobial agents [3–9, 16, 25–29]. Multi-drug resistance and inappropriate antimicrobial treatment along with the severe underlying disease and comorbidities of the patients may, at least in part, result in the high morbidity and mortality observed in ICU patients who develop an infection [5, 7–10, 15, 30].
Acinetobacter baumannii has emerged as a significant cause of infection in SICUs and ICUs in general [2, 4–6, 8–16, 25, 26, 28–33]. It is a widespread, environmental, opportunistic, non-fermentative, aerobic gram-negative coccobacillus that has minimal nutritional requirements and can survive on a wide variety of surfaces and environments. This organism can form part of the bacterial flora of the skin, respiratory, and gastrointestinal tract, is easily transmitted, and is able to remain viable in a hospital environment for a long time [4, 9, 16]. All these factors have contributed to the increased Acinetobacter baumannii nosocomial infections incidence [4, 9, 16]. Nosocomial spread of Acinetobacter baumannii in the ICU setting often is attributed to colonized inanimate environment, equipment or devices, and personnel [4, 6, 9, 10, 16, 34]. Moreover, it is becoming increasingly resistant to antibiotics and associated with high mortality [4–6, 8–12, 15, 16, 26–33, 35].
Infections caused by this pathogen are difficult to control and treat because of its high resistance in the environment and ability to develop rapidly multiple antibiotic resistance. Given its increasing resistance rates, multi-drug resistance can be expected to become more prevalent in many hospitals. Therefore, the potential for antimicrobial resistance is an important concern for clinicians treating patients with Acinetobacter baumannii infections. The objective of this study was to evaluate the incidence of Acinetobacter baumannii infections in our SICU along with the clinical features and outcome of these patients and, moreover, to investigate the incidence of multi-resistant Acinetobacter baumannii and its antimicrobial susceptibility. Our particular goal was to identify potential risk factors and predictors of multi-drug resistance.
In agreement with the literature, the incidence of Acinetobacter baumannii infection in the patients who were hospitalized in our unit was quite high (12.6%) [2, 6, 8, 10, 13–15, 25]. Recorded infection sites were bloodstream, respiratory tract, surgical site, central venous catheter, and urinary tract [2, 4, 6, 8–14, 16, 28, 32]; bloodstream and respiratory tract infections represented the majority of infections in our patients [2, 4, 6, 8–16, 28, 35].
Prolonged SICU and hospital stay as well as high morbidity and mortality of the patients was identified. Comparison of the patients with Acinetobacter baumannii infection with those with infection caused by all other organisms revealed that Acinetobacter infection was associated with a significantly higher morbidity and mortality. However, given the debilitated physical condition of these patients, it is difficult to distinguish morbidity and mortality attributable to these infections from morbidity and mortality due to severity of underlying disease and comorbidities. Several studies have suggested that Acinetobacter baumannii infections in ICU patients are associated with prolonged hospital stay and high morbidity and mortality [4–16, 28, 30–33, 35].
In addition, significant resistance to the majority of antibiotics was observed as well as high incidence of multi-drug resistant Acinetobacter baumannii. Colistin was the only effective antimicrobial agent (100% susceptibility) in our cases. Although differences regarding antimicrobial resistance pattern of Acinetobacter baumannii infections are identified, high resistance rates to almost all available antibiotics are noticed in several studies, thus limiting treatment options [4–6, 8, 9, 11–16, 26–30, 33–36]. Furthermore, a substantial share of these reported isolates are susceptible only to colistin. In accordance with our results, resistance to colistin is infrequent [5, 13, 27–29, 34, 35]. The safety and efficacy of colistin treatment in Acinetobacter baumannii infections has been reported in other studies [13, 27, 35]. The development of new agents, the reappraisal of older compounds, such as polymyxins, and combination therapy are essential for the optimal treatment of these multi–drug-resistant organisms [4, 5, 13, 16, 26–29, 35]. Moreover, antibiotic resistance is a major risk factor for epidemic behavior of Acinetobacter baumannii [37].
Morbidity and mortality in the multi-drug-resistant group were much higher than in the non-multi-drug-resistant group. The difference in morbidity was statistically significant. Furthermore, although this difference did not marginally reach statistical significance (p = 0.06), mortality in the multi-resistant group was almost double than that of the non-multi-resistant group (60.8% vs. 31.1%). These results have an important bearing on the effect of multi-drug-resistant Acinetobacter infections on patients’ outcome and on the significance of the identification of predictive factors of such a resistance for prevention and treatment.
In this study, univariate analysis of risk factors for multi-resistance revealed a significant association with the number of previous antibiotics, previous aminoglycoside administration, previous septic shock, previous ARDS, and reoperation. Multivariate analysis showed that previous septic shock and reoperation are independent predictors of Acinetobacter baumannii multi-drug resistance.
Age, APACHE II score, hospital size, previous ICU stay and length of ICU stay, high concentration of patients with carbapenem-resistant Acinetobacter baumannii in the same ICU ward, previous infection, previous surgical treatment, previous carriage of carbapenem-resistant Acinetobacter baumannii, arterial or venous catheter, presence and duration of intubation/tracheostomy, presence and days with urinary catheter, nasogastric tube and parenteral nutrition, number of previous antibiotics prescribed, and previous antimicrobial therapy (particularly with cephalosporins, aminoglycosides, carbapenems, or vancomycin) have been recognised as risk factors for infection or colonisation by resistant Acinetobacter baumannii [6, 12, 28–30, 38]. Among them, in multivariate analysis APACHE II, hospital size ( > 500 beds), previous infection, high density of patients with carbapenem-resistant Acinetobacter baumannii in the same ICU ward, previous carriage of carbapenem-resistant Acinetobacter baumannii, presence of arterial or urinary catheter, previous surgery, and previous antibiotic treatment (especially with carbapenems) were independent predisposing factors [6, 12, 28, 30]. Additionally, Abbo et al [11] reported home antibiotic treatment, male gender, ischemic heart disease, and mechanical ventilation as significant risk factors for multi-resistant Acinetobacter baumanii infection. In another study, the use of antecedent antibacterials and intubation were found to be independent predictors of multi–drug-resistant Acinetobacter baumanii infections (susceptible only to colistin) [36]. Age, previous ICU stay, previous antimicrobial therapy (exposure to third-generation cephalosporins, imipenem, vancomycin, quinolones, and aminoglycosides), previous surgery, previous sepsis, mechanical ventilation, and urinary tract catheter also have been reported as predisposing factors for Acinetobacter baumannii nosocomial infections in critically ill patients [5, 13, 31, 33, 39]. Presence of ARDS and previous antibiotic use also have been identified as independent variables correlated with the development of ventilator-associated Acinetobacter baumannii pneumonia [32, 33], whereas previous episode of sepsis, exposure to antibiotics before infection (particularly imipenem), reintubation, and length of mechanical ventilation and hospital stay before infection have been identified as risk factors for ventilator-associated multi-resistant (susceptible exclusively to colistin) Acinetobacter baumannii pneumonia [33]. In addition, previous antimicrobial therapy, previous infection, operation, and APACHE II have been associated with ICU-acquired infections in general [2, 25]. Moreover, the number of antibiotics administered before the onset of infection as well as previous use of broad-spectrum cephalosporins and aminoglycosides have been identified as significant risk factors for multi–drug-resistant pseudomonas aeruginosa [7]. Interestingly, although previous surgery has been reported as a risk factor for multi-resistant Acinetobacter baumannii, reoperation has not been evaluated in any study.
The high incidence of reduced antibiotic susceptibility among bacteria in ICUs suggests that more effective strategies are needed to control the selection and spread of resistant organisms [4–9, 12, 16, 26, 29]. Organization of a hospital infections prevention and control program in worldwide and national level and in every ICU is of utmost importance to reduce their incidence, prevent microbial multi-resistance, reduce hospital costs, and improve patients’ prognosis [1, 2, 5–7, 9, 12, 16, 26, 29].
There are several main causes of Acinetobacter baumannii nosocomial infections and drug resistance in the ICU: poor compliance with hygiene regimes, inadequate disinfection or sterilization of mattresses, bed sheets, dressing material, devices and other equipment, prolonged catheterization with central and peripheral lines and urinary and nasogastric/nasointestinal catheters, inefficient isolation of infected patients, overcrowding, prolonged mechanical ventilation and hospitalization, decreased host resistance, and inappropriate antibiotic use [4–6, 8–12, 14, 16, 26, 28, 31, 33, 34, 39]. A combination of appropriate and adequate control measures has become increasingly important in efforts to contain the spread of this organism. Strict hand washing before and after handling patients and routine use of gloves should be implemented, and strategies to prevent medical device-related infection and cross-infection in the hospital are of utmost importance [4–6, 8–10, 12, 16, 26, 28, 34, 39]. Overcrowding must be avoided, and preemptive isolation of all patients with risk factors for infection by resistant organisms would likely reduce secondary spread within the hospital [6, 16, 26]. Optimization of antimicrobial use is recommended [4–6, 8–10, 12, 16, 26, 28, 29, 31, 33, 34, 39]. Restricted antibiotic-use policy may result in a reduction in costs linked to antibiotics and selective reduction of nosocomial infections due to antimicrobial resistant microorganisms. Educational programs and institutional guidelines on the basis of local needs and judgments should be established.
The lack of any new compounds in the near future indicates that national, regional, and local surveillance efforts are imperative to provide clinicians with information for choosing empirical or directed therapy. Continued surveillance of microorganisms and antimicrobial agents in every ICU seems warranted to monitor the occurrence and spread of antimicrobial resistance in pathogens causing infections and the possible emergence of resistance mechanisms that could compromise empiric therapy [4–6, 16, 26]. Monitoring and optimization of antimicrobial use also is strictly recommended [5, 6, 16, 26, 29].
Because each hospital unit has a distinct bacteriological profile and antibiotic resistance pattern, knowledge of these differences is critical for epidemiological surveillance. This information is very helpful for planning effective therapy against microorganisms and reducing overall infection-related costs, morbidity, and mortality [5, 6, 26]. Empiric treatment regimens should be based on unit-specific data [5, 6, 26]. Therefore, local resistance surveillance programs are valuable for developing appropriate therapeutic guidelines for specific infections and patient types.
The clinical features and presentation of an Acinetobacter infection are non-specific. Because this organism usually causes ICU-acquired infections in severely ill ICU patients, as shown in our study by the high mean APACHE II and SOFA scores of the patients, a high index of suspicion in these patients when presenting clinical signs of an ICU-acquired infection is imperative, although it is difficult to suspect such an infection clinically. In addition, although some of them are common for several microorganisms, the presence of known predisposing factors for an Acinetobacter baumannii infection, such as advanced age, prolonged hospital and ICU stay, use of broad-spectrum antibiotics, prolonged mechanical ventilation or utilization of arterial and venous lines, urinary and nasogastric catheters, recent operation, previous ARDS, and previous episode of sepsis is very helpful for suspicion of such an infection. Moreover, high concentration of patients with Acinetobacter baumannii in the same ICU ward and history of previous Acinetobacter colonization or infection in the same patient make the diagnosis of such an infection very possible. Finally, knowledge of the bacteriological profile of a specific ICU is particularly important, enhancing the clinical suspicion of the infection in ICUs with a high incidence of Acinetobacter infections.
Prompt, appropriate treatment of Acinetobacter infections is of the utmost importance because delayed or inadequate treatment is associated with high morbidity and mortality in these patients [5, 9, 15, 33]. Therefore, treatment should be initiated immediately in patients presenting clinical signs of infection, particularly when there is a high suspicion of Acinetobacter infection in severely ill ICU patients and should be based on unit-specific data regarding the antibiotic resistance pattern of the organism in the specific ICU.
Conclusion
Acinetobacter baumannii infections affect frequently severely ill patients in SICUs and are associated with high morbidity, mortality, and multi-resistance. Eradication of this pathogen requires implementation of rigorous infection control practices and effective antimicrobial therapy. Our results showed that multi-drug resistance may be associated with worse outcome; therefore, recognition of clinical and microbiological features of these patients and identification of potential multi-resistance risk factors are essential for prevention and treatment of infections. Avoidance of unnecessary antibiotics should be a high priority for the management of these patients, whereas specific attention should be paid to treating ARDS. Moreover, because previous septic shock and reoperation were identified as independent predictors of multi-resistance, great caution should be used when patients have such factors. When such risk factors are identified, colistin may be the only appropriate treatment. Unfortunately, the unique propensity of this organism to develop resistance to multiple antimicrobial agents reinforces concerns about the imminence of a post-antimicrobial era in which no effective antibiotics will be available to treat this type of infection.
References
Vincent JL, Bihari DJ, Suter PM et al (1995) The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 274:639–644
Erbay H, Yalcin AN, Serin S et al (2003) Nosocomial infections in intensive care unit in a Turkish university hospital: a 2-year survey. Intensive Care Med 29:1482–1488
Fridkin SK, Steward CD, Edwards JR et al (1999) Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis 29:245–252
Van Looveren M, Goossens H, ARPAC Steering Group (2004) Antimicrobial resistance of Acinetobacter spp. in Europe. Clin Microbiol Infect 10:684–704
Gomez J, Simarro E, Banos V et al (1999) Six-year prospective study of risk and prognostic factors in patients with nosocomial sepsis caused by Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 18:358–361
Corbella X, Montero A, Pujol M et al (2000) Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol 38:4086–4095
Aloush V, Navon-Venezia S, Seigman-Igra Y et al (2006) Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 50:43–48
Seifert H, Strate A, Pulverer G (1995) Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore) 74:340–349
Cisneros JM, Rodriguez-Bano J (2002) Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect 8:687–693
Villari P, Iacuzio L, Vozzella EA et al (1999) Unusual genetic heterogeneity of Acinetobacter baumannii isolates in a university hospital in Italy. Am J Infect Control 27:247–253
Abbo A, Navon-Venezia S, Hammer-Muntz O et al (2005) Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis 11:22–29
Cisneros JM, Rodriguez-Bano J, Fernandez-Cuenca F et al (2005) Risk factors for the acquisition of imipenem-resistant Acinetobacter baumannii in Spain: a nationwide study. Clin Microbiol Infect 11:874–879
Reina R, Estenssoro E, Saenz G et al (2005) Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med 31:1058–1065
Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F et al (2004) Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 25:819–824
Cisneros JM, Reyes MJ, Pachón J et al (1996) Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis 22:1026–1032
Bergogne-Bérézin E, Towner KJ (1996) Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev 9:148–165
Garner JS, Jarvis WR, Emori TG et al (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
Horan TC, Gaynes RP, Martone WJ et al (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 20:271–274
Calandra T, Cohen J (2005) International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 33:1538–1548
National Committee for Clinical Laboratory Standards (2002) Performance standards for antimicrobial susceptibility testing; 11th informational supplement. National Committee for Clinical Laboratory Standards. Pennsylvania, Wayne 22:M100-S12
Knaus WA, Draper EA, Wagner DP et al (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Levy MM, Fink MP, Marshall JC et al (2003) SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1256
Meric M, Willke A, Caglayan C et al (2005) Intensive care unit-acquired infections: incidence, risk factors and associated mortality in a Turkish university hospital. Jpn J Infect Dis 58:297–302
Rello J (1999) Acinetobacter baumannii infections in the ICU: customization is the key. Chest 115:1226–1229
Li J, Nation RL, Milne RW et al (2005) Evaluation of colistin as an agent against mutli-resistant Gram-negative bacteria. Int J Antimicrob Agents 25:11–25
del Mar Tomas M, Cartelle M, Pertega S et al (2005) Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk-factors for colonisation and infection. Clin Microbiol Infect 11:540–546
Manikal VM, Landman D, Saurina G et al (2000) Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis 31:101–106
Lortholary O, Fagon JY, Hoi AB et al (1995) Nosocomial acquisition of multiresistant Acinetobacter baumannii: risk factors and prognosis. Clin Infect Dis 20:790–796
Lee SO, Kim NJ, Choi SH et al (2004) Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case-control study. Antimicrob Agents Chemother 48:224–228
Baraibar J, Correa H, Mariscal D et al (1997) Risk factors for infection by Acinetobacter baumannii in intubated patients with nosocomial pneumonia. Chest 112:1050–1054
Garnacho-Montero J, Ortiz-Leyba C, Fernández-Hinojosa E et al (2005) Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med 31:649–655
Go ES, Urban C, Burns J et al (1994) Clinical and molecular epidemiology of Acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet 344:1329–1332
Michalopoulos AS, Tsiodras S, Rellos K et al (2005) Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect 11:115–121
Levin AS, Mendes CM, Sinto SI et al (1996) An outbreak of multiresistant Acinetobacter baumanii in a university hospital in Sao Paulo, Brazil. Infect Control Hosp Epidemiol 17:366–368
Koeleman JG, van der Bijl MW, Stoof J et al (2001) Antibiotic resistance is a major risk factor for epidemic behavior of Acinetobacter baumannii. Infect Control Hosp Epidemiol 22:284–288
Koeleman JG, Parlevliet GA, Dijkshoorn L et al (1997) Nosocomial outbreak of multi-resistant Acinetobacter baumannii on a surgical ward: epidemiology and risk factors for acquisition. J Hosp Infect 37:113–123
Garcia-Garmendia JL, Ortiz-Leyba C, Garnacho-Montero J et al (2001) Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically ill patients: a cohort study. Clin Infect Dis 33:939–946
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katsaragakis, S., Markogiannakis, H., Toutouzas, K.G. et al. Acinetobacter baumannii Infections in a Surgical Intensive Care Unit: Predictors of Multi-drug Resistance. World J Surg 32, 1194–1202 (2008). https://doi.org/10.1007/s00268-008-9571-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-008-9571-3