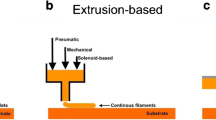
Abstract
Organoids are 3D structures generated from stem cells. Their functions and physiological characteristics are similar to those of normal organs. They are used in disease mechanism research, new drug development, organ transplantation and other fields. In recent years, the application of 3D materials in plastic surgery for repairing injuries, filling, tissue reconstruction and regeneration has also been investigated. The PubMed/MEDLINE database was queried to search for animal and human studies published through July of 2022 with search terms related to Organoids, Plastic Surgery, Pluripotent Stem Cells, Bioscaffold, Skin Reconstruction, Bone and Cartilage Regeneration. This review presents stem cells, scaffold materials and methods for the construction of organoids for plastic surgery, and it summarizes their research progress in plastic surgery in recent years.
Level of Evidence III This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.


Similar content being viewed by others
References
Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B (2020) Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 12:1
Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, Upponi SS, Brevini T, Wesley BT, Garcia-Bernardo J, Mahbubani K, Canu G, Gieseck R 3rd, Berntsen NL, Mulcahy VL, Crick K, Fear C, Robinson S, Swift L, Gambardella L, Bargehr J, Ortmann D, Brown SE, Osnato A, Murphy MP, Corbett G, Gelson WTH, Mells GF, Humphreys P, Davies SE, Amin I, Gibbs P, Sinha S, Teichmann SA, Butler AJ, See TC, Melum E, Watson CJE, Saeb-Parsy K, Vallier L (2021) Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 371:839–846
Zanoni M, Cortesi M, Zamagni A, Arienti C, Pignatta S, Tesei A (2020) Modeling neoplastic disease with spheroids and organoids. J Hematol Oncol 13:97
Clevers H (2016) Modeling Development and Disease with Organoids. Cell 165:1586–1597
Kimbrel EA, Lanza R (2020) Next-generation stem cells - ushering in a new era of cell-based therapies. Nat Rev Drug Discov 19:463–479
Rodriguez-Polo I, Behr R (2022) Non-human primate pluripotent stem cells for the preclinical testing of regenerative therapies. Neural Regen Res 17:1867–1874
Cao S, Loh K, Pei Y, Zhang W, Han J (2012) Overcoming barriers to the clinical utilization of iPSCs: reprogramming efficiency, safety and quality. Protein Cell 3:834–845
Andrzejewska A, Lukomska B, Janowski M (2019) Concise review: mesenchymal stem cells: from roots to boost. Stem Cells 37:855–864
Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33:1402–1416
Zhang R, Ma J, Han J, Zhang W, Ma J (2019) Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res 11:6275–6289
Contentin R, Jammes M, Bourdon B, Casse F, Bianchi A, Audigie F, Branly T, Velot E, Galera P (2022) Bone marrow MSC secretome increases equine articular chondrocyte collagen accumulation and their migratory capacities. Int J Mol Sci 23:1
Wang T, Xu W, Zhao X, Bai B, Hua Y, Tang J, Chen F, Liu Y, Wang Y, Zhou G, Cao Y (2022) Repair of osteochondral defects mediated by double-layer scaffolds with natural osteochondral-biomimetic microenvironment and interface. Mater Today Bio 14:100234
Ma K, Laco F, Ramakrishna S, Liao S, Chan CK (2009) Differentiation of bone marrow-derived mesenchymal stem cells into multi-layered epidermis-like cells in 3D organotypic coculture. Biomaterials 30:3251–3258
Xiang J, Zhou L, Xie Y, Zhu Y, Xiao L, Chen Y, Zhou W, Chen D, Wang M, Cai L, Guo L (2022) Mesh-like electrospun membrane loaded with atorvastatin facilitates cutaneous wound healing by promoting the paracrine function of mesenchymal stem cells. Stem Cell Res Ther 13:190
Silini AR, Cargnoni A, Magatti M, Pianta S, Parolini O (2015) The long path of human placenta, and its derivatives, in regenerative medicine. Front Bioeng Biotechnol 3:162
Rousselle P, Braye F, Dayan G (2019) Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev 146:344–365
Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, Bahrami S, Niknejad H (2019) Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front Immunol 10:238
Farhadihosseinabadi B, Farahani M, Tayebi T, Jafari A, Biniazan F, Modaresifar K, Moravvej H, Bahrami S, Redl H, Tayebi L, Niknejad H (2018) Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif Cells Nanomed Biotechnol 46:431–440
Yu SC, Xu YY, Li Y, Xu B, Sun Q, Li F, Zhang XG (2015) Construction of tissue engineered skin with human amniotic mesenchymal stem cells and human amniotic epithelial cells. Eur Rev Med Pharmacol Sci 19:4627–4635
Pan C, Lang H, Zhang T, Wang R, Lin X, Shi P, Zhao F, Pang X (2019) Conditioned medium derived from human amniotic stem cells delays H2O2induced premature senescence in human dermal fibroblasts. Int J Mol Med 44:1629–1640
Liu QW, Huang QM, Wu HY, Zuo GS, Gu HC, Deng KY, Xin HB (2021) Characteristics and therapeutic potential of human amnion-derived stem cells. Int J Mol Sci 22:1
Cao L, Liu W, Zhong Y, Zhang Y, Gao D, He T, Liu Y, Zou Z, Mo Y, Peng S, Shuai C (2020) Linc02349 promotes osteogenesis of human umbilical cord-derived stem cells by acting as a competing endogenous RNA for miR-25-3p and miR-33b-5p. Cell Prolif 53:e12814
Yue C, Guo Z, Luo Y, Yuan J, Wan X, Mo Z (2020) c-Jun overexpression accelerates wound healing in diabetic rats by human umbilical cord-derived mesenchymal stem cells. Stem Cells Int 2020:7430968
Uzbas F, May ID, Parisi AM, Thompson SK, Kaya A, Perkins AD, Memili E (2015) Molecular physiognomies and applications of adipose-derived stem cells. Stem Cell Rev Rep 11:298–308
Al-Ghadban S, Diaz ZT, Singer HJ, Mert KB, Bunnell BA (2020) Increase in leptin and PPAR-gamma gene expression in lipedema adipocytes differentiated in vitro from adipose-derived stem cells. Cells 9:1
Yuan B, Broadbent JA, Huan J, Yang H (2018) The effects of adipose stem cell-conditioned media on fibrogenesis of dermal fibroblasts stimulated by transforming growth factor-beta1. J Burn Care Res 39:129–140
An R, Zhang Y, Qiao Y, Song L, Wang H, Dong X (2020) Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice. Stem Cell Res Ther 11:120
Liu F, Wang X, Li Y, Ren M, He P, Wang L, Xu J, Yang S, Ji P (2022) Dendrimer-modified gelatin methacrylate hydrogels carrying adipose-derived stromal/stem cells promote cartilage regeneration. Stem Cell Res Ther 13:26
Bicer M, Sheard J, Iandolo D, Boateng SY, Cottrell GS, Widera D (2020) Electrical stimulation of adipose-derived stem cells in 3D nanofibrillar cellulose increases their osteogenic potential. Biomolecules 10:1
Zhang Y, Yang Y, Jiang M, Huang SX, Zhang W, Al Alam D, Danopoulos S, Mori M, Chen YW, Balasubramanian R, De Sousa C, Lopes SM, Serra C, Bialecka M, Kim E, Lin S, Toste De Carvalho ALR, Riccio PN, Cardoso WV, Zhang X, Snoeck HW, Que J (2018) 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell 23(516–529):e5
Adel IM, Elmeligy MF, Elkasabgy NA (2022) Conventional and recent trends of scaffolds fabrication: a superior mode for tissue engineering. Pharmaceutics 14:1
Kleinman HK, Martin GR (2005) Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15:378–386
Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, Lewis M, Stelzner M, Martin MG, Dunn JC (2014) Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS ONE 9:e107814
Xi W, Saleh J, Yamada A, Tomba C, Mercier B, Janel S, Dang T, Soleilhac M, Djemat A, Wu H, Romagnolo B, Lafont F, Mege RM, Chen Y, Delacour D (2022) Modulation of designer biomimetic matrices for optimized differentiated intestinal epithelial cultures. Biomaterials 282:121380
Shubin AD, Felong TJ, Schutrum BE, Joe DSL, Ovitt CE, Benoit DSW (2017) Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater 50:437–449
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243
Fu RH, Wang YC, Liu SP, Shih TR, Lin HL, Chen YM, Sung JH, Lu CH, Wei JR, Wang ZW, Huang SJ, Tsai CH, Shyu WC, Lin SZ (2014) Decellularization and recellularization technologies in tissue engineering. Cell Transplant 23:621–630
Ibsirlioglu T, Elcin AE, Elcin YM (2020) Decellularized biological scaffold and stem cells from autologous human adipose tissue for cartilage tissue engineering. Methods 171:97–107
Seliktar D (2012) Designing cell-compatible hydrogels for biomedical applications. Science 336:1124–1128
Akther F, Little P, Li Z, Nguyen NT, Ta HT (2020) Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv 10:43682–43703
Vanderburgh J, Sterling JA, Guelcher SA (2017) 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann Biomed Eng 45:164–179
Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, Johansson F, Randles A, Rosenkrantz JE, Louis-Rosenberg JD, Galie PA, Stevens KR, Miller JS (2019) Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364:458–464
Trivedi P, Liu R, Bi H, Xu C, Rosenholm JM, Akerfelt M (2021) 3D modeling of epithelial tumors-the synergy between materials engineering, 3D bioprinting, high-content imaging, and nanotechnology. Int J Mol Sci 22:1
Singh S, Choudhury D, Yu F, Mironov V, Naing MW (2020) In situ bioprinting—Bioprinting from benchside to bedside? Acta Biomater 101:14–25
Feng Q, Xu J, Zhang K, Yao H, Zheng N, Zheng L, Wang J, Wei K, Xiao X, Qin L, Bian L (2019) Dynamic and cell-infiltratable hydrogels as injectable carrier of therapeutic cells and drugs for treating challenging bone defects. ACS Cent Sci 5:440–450
Liu Y, Peng L, Li L, Huang C, Shi K, Meng X, Wang P, Wu M, Li L, Cao H, Wu K, Zeng Q, Pan H, Lu WW, Qin L, Ruan C, Wang X (2021) 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials 279:121216
Kabirian F, Mela P, Heying R (2022) 4D printing applications in the development of smart cardiovascular implants. Front Bioeng Biotechnol 10:873453
Ghazal AF, Zhang M, Mujumdar AS, Ghamry M (2022) Progress in 4D/5D/6D printing of foods: applications and R&D opportunities. Crit Rev Food Sci Nutr 1:1–24
Okano T, Yamada N, Sakai H, Sakurai Y (1993) A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res 27:1243–1251
Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T (2001) Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng 7:473–480
Shimizu T, Yamato M, Akutsu T, Shibata T, Isoi Y, Kikuchi A, Umezu M, Okano T (2002) Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res 60:110–117
Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, Okano T (2004) Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 77:379–385
Takagi S, Ohno M, Ohashi K, Utoh R, Tatsumi K, Okano T (2012) Cell shape regulation based on hepatocyte sheet engineering technologies. Cell Transplant 21:411–420
Antoni D, Burckel H, Josset E, Noel G (2015) Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 16:5517–5527
Sharma A, Janus JR, Hamilton GS (2015) Regenerative medicine and nasal surgery. Mayo Clin Proc 90:148–158
Schon BS, Hooper GJ, Woodfield TB (2017) Modular Tissue Assembly Strategies for Biofabrication of Engineered Cartilage. Ann Biomed Eng 45:100–114
Schubert T, Anders S, Neumann E, Scholmerich J, Hofstadter F, Grifka J, Muller-Ladner U, Libera J, Schedel J (2009) Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. Int J Mol Med 23:455–460
Ingber DE (2022) Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet 1:1–25
Esch EW, Bahinski A, Huh D (2015) Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14:248–260
Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA (2021) Organs-on-chips: into the next decade. Nat Rev Drug Discov 20:345–361
Occhetta P, Mainardi A, Votta E, Vallmajo-Martin Q, Ehrbar M, Martin I, Barbero A, Rasponi M (2019) Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat Biomed Eng 3:545–557
Mondadori C, Palombella S, Salehi S, Talo G, Visone R, Rasponi M, Redaelli A, Sansone V, Moretti M, Lopa S (2021) Recapitulating monocyte extravasation to the synovium in an organotypic microfluidic model of the articular joint. Biofabrication 13:1
Da LC, Huang YZ, Xie HQ, Zheng BH, Huang YC, Du SR (2021) Membranous extracellular matrix-based scaffolds for skin wound healing. Pharmaceutics 13:1
Martino F, Perestrelo AR, Vinarsky V, Pagliari S, Forte G (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9:824
Ramadan Q, Ting FC (2016) In vitro micro-physiological immune-competent model of the human skin. Lab Chip 16:1899–1908
Mori N, Morimoto Y, Takeuchi S (2017) Skin integrated with perfusable vascular channels on a chip. Biomaterials 116:48–56
Cavalcante Pita Neto I, Franco MPL, J, Chaves Moreno E, Pita P, Aurelio Lucchesi Sandrini F and Gomes De Alencar Gondim D, (2018) A Patient With Severe Lower Face Degloving Injury. J Craniofac Surg 29:e608–e610
Proksch E, Brandner JM, Jensen JM (2008) The skin: an indispensable barrier. Exp Dermatol 17:1063–1072
Itoh M, Umegaki-Arao N, Guo Z, Liu L, Higgins CA, Christiano AM (2013) Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS ONE 8:e77673
Ebner-Peking P, Krisch L, Wolf M, Hochmann S, Hoog A, Vari B, Muigg K, Poupardin R, Scharler C, Schmidhuber S, Russe E, Stachelscheid H, Schneeberger A, Schallmoser K, Strunk D (2021) Self-assembly of differentiated progenitor cells facilitates spheroid human skin organoid formation and planar skin regeneration. Theranostics 11:8430–8447
Lee J, Bscke R, Tang PC, Hartman BH, Heller S, Koehler KR (2018) Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep 22:242–254
Bahmad HF, Poppiti R, Alexis J (2021) Nanotherapeutic approach to treat diabetic foot ulcers using tissue-engineered nanofiber skin substitutes: a review. Diabetes Metab Syndr 15:487–491
Tiku ML, Sabaawy HE (2015) Cartilage regeneration for treatment of osteoarthritis: a paradigm for nonsurgical intervention. Ther Adv Musculoskelet Dis 7:76–87
Marchan J, Wittig O, Diaz-Solano D, Gomez M, Cardier JE (2022) Enhanced chondrogenesis from chondrocytes co-cultured on mesenchymal stromal cells: Implication for cartilage repair. Injury 53:399–407
Spasovski D, Spasovski V, Bascarevic Z, Stojiljkovic M, Vreca M, Andelkovic M, Pavlovic S (2018) Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med 20:1
Li Q, Zhao F, Li Z, Duan X, Cheng J, Zhang J, Fu X, Zhang J, Shao Z, Guo Q, Hu X, Ao Y (2020) Autologous fractionated adipose tissue as a natural biomaterial and novel one-step stem cell therapy for repairing articular cartilage defects. Front Cell Dev Biol 8:694
Nguyen HT, Vu NB (2021) A simple method to produce engineered cartilage from human adipose-derived mesenchymal stem cells and poly epsilon-caprolactone scaffolds. Adv Exp Med Biol. https://doi.org/10.1007/5584_2021_669
Zhou G, Jiang H, Yin Z, Liu Y, Zhang Q, Zhang C, Pan B, Zhou J, Zhou X, Sun H, Li D, He A, Zhang Z, Zhang W, Liu W, Cao Y (2018) In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine 28:287–302
Al-Ghadban S, Artiles M, Bunnell BA (2021) Adipose stem cells in regenerative medicine: looking forward. Front Bioeng Biotechnol 9:837464
Baddour JA, Sousounis K, Tsonis PA (2012) Organ repair and regeneration: an overview. Birth Defects Res C Embryo Today 96:1–29
Yanai R, Tetsuo F, Ito S, Itsumi M, Yoshizumi J, Maki T, Mori Y, Kubota Y, Kajioka S (2019) Extracellular calcium stimulates osteogenic differentiation of human adipose-derived stem cells by enhancing bone morphogenetic protein-2 expression. Cell Calcium 83:102058
Forrestal DP, Klein TJ, Woodruff MA (2017) Challenges in engineering large customized bone constructs. Biotechnol Bioeng 114:1129–1139
Mohiuddin OA, Campbell B, Poche JN, Ma M, Rogers E, Gaupp D, MaA H, Bunnell BA, Hayes DJ, Gimble JM (2019) Decellularized adipose tissue hydrogel promotes bone regeneration in critical-sized mouse femoral defect model. Front Bioeng Biotechnol 7:211
Liu B, Li X, Qiu W, Liu Z, Zhou F, Zheng Y, Wen P, Tian Y (2022) Mechanical distribution and new bone regeneration after implanting 3D printed prostheses for repairing metaphyseal bone defects: a finite element analysis and prospective clinical study. Front Bioeng Biotechnol 10:921545
Boffa A, Solaro L, Poggi A, Andriolo L, Reale D, Di Martino A (2021) Multi-layer cell-free scaffolds for osteochondral defects of the knee: a systematic review and meta-analysis of clinical evidence. J Exp Orthop 8:56
Abedin Dargoush S, Hanaee-Ahvaz H, Irani S, Soleimani M, Khatami SM, Sohi AN (2022) A composite bilayer scaffold functionalized for osteochondral tissue regeneration in rat animal model. J Tissue Eng Regen Med 16:559–574
Willemse J, Roos FJM, Voogt IJ, Schurink IJ, Bijvelds M, De Jonge HR, Van Der Laan LJW, De Jonge J, Verstegen MMA (2021) Scaffolds obtained from decellularized human extrahepatic bile ducts support organoids to establish functional biliary tissue in a dish. Biotechnol Bioeng 118:836–851
Robertson MJ, Soibam B, O’leary JG, Sampaio LC and Taylor DA, (2018) Recellularization of rat liver: an in vitro model for assessing human drug metabolism and liver biology. PLoS ONE 13:e0191892
Maqueda M, Mosquera JL, Garcia-Arumi J, Veiga A, Duarri A (2021) Repopulation of decellularized retinas with hiPSC-derived retinal pigment epithelial and ocular progenitor cells shows cell engraftment, organization and differentiation. Biomaterials 276:121049
Bhattacharjee M, Ivirico JLE, Kan HM, Bordett R, Pandey R, Otsuka T, Nair LS, Laurencin CT (2020) Preparation and characterization of amnion hydrogel and its synergistic effect with adipose derived stem cells towards IL1beta activated chondrocytes. Sci Rep 10:18751
Wu X, He L, Li W, Li H, Wong WM, Ramakrishna S, Wu W (2017) Functional self-assembling peptide nanofiber hydrogel for peripheral nerve regeneration. Regen Biomater 4:21–30
Lovett M, Lee K, Edwards A, Kaplan DL (2009) Vascularization strategies for tissue engineering. Tissue Eng Part B Rev 15:353–370
Citro A, Moser PT, Dugnani E, Rajab TK, Ren X, Evangelista-Leite D, Charest JM, Peloso A, Podesser BK, Manenti F, Pellegrini S, Piemonti L, Ott HC (2019) Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials 199:40–51
Grebenyuk S, Ranga A (2019) Engineering Organoid Vascularization. Front Bioeng. Biotechnol 7:39
Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA (2016) Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 113:3179–3184
Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R (2019) Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16:255–262
Worsdorfer P, Rockel A, Alt Y, Kern A, Ergun S (2020) Generation of vascularized neural organoids by co-culturing with mesodermal progenitor cells. STAR Protoc 1:100041
Shelkey E, Oommen D, Stirling ER, Soto-Pantoja DR, Cook KL, Lu Y, Votanopoulos KI, Soker S (2022) Immuno-reactive cancer organoid model to assess effects of the microbiome on cancer immunotherapy. Sci Rep 12:9983
Bironzo P, Primo L, Novello S, Righi L, Candeloro S, Manganaro L, Bussolino F, Pirri F and Scagliotti GV (2022) Clinical-molecular prospective cohort study in non-small cell lung cancer (PROMOLE study): a comprehensive approach to identify new predictive markers of pharmacological response. Clin Lung Cancer 23:e347–e352
Feier AM, Portan D, Manu DR, Kostopoulos V, Kotrotsos A, Strnad G, Dobreanu M, Salcudean A, Bataga T (2022) Primary MSCs for personalized medicine: ethical challenges, isolation and biocompatibility evaluation of 3D electrospun and printed scaffolds. Biomedicines 10:1
Van Winkle LJ, Ryznar RJ, Iannaccone PM (2022) Editorial: from single stem cells to organoids, organ repair, and public health. Front Cell Dev Biol 10:849889
Bi H, Karanth SS, Ye K, Stein R, Jin S (2020) Decellularized tissue matrix enhances self-assembly of islet organoids from pluripotent stem cell differentiation. ACS Biomater Sci Eng 6:4155–4165
Gathani KM, Raghavendra SS (2016) Scaffolds in regenerative endodontics: a review. Dent Res J (Isfahan) 13:379–386
Ye J, Xiao J, Wang J, Ma Y, Zhang Y, Zhang Q, Zhang Z, Yin H (2021) The interaction between intracellular energy metabolism and signaling pathways during osteogenesis. Front Mol Biosci 8:807487
Diaz-Prado S, Muinos-Lopez E, Hermida-Gomez T, Rendal-Vazquez ME, Fuentes-Boquete I, De Toro FJ, Blanco FJ (2010) Multilineage differentiation potential of cells isolated from the human amniotic membrane. J Cell Biochem 111:846–857
Zeng N, Chen H, Wu Y, Liu Z (2021) Adipose stem cell-based treatments for wound healing. Front Cell Dev Biol 9:821652
Bouland C, Philippart P, Dequanter D, Corrillon F, Loeb I, Bron D, Lagneaux L, Meuleman N (2021) Cross-talk between mesenchymal stromal cells (MSCs) and endothelial progenitor cells (EPCs) in bone regeneration. Front Cell Dev Biol 9:674084
Funding
This work was supported by the National Nature Science Foundation of China (No. 81873939)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, H., Wang, G., Shang, Y. et al. Organoids and Their Research Progress in Plastic and Reconstructive Surgery. Aesth Plast Surg 47, 880–891 (2023). https://doi.org/10.1007/s00266-022-03129-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-022-03129-6