Abstract
Background
The use of injectable hyaluronic acid–based gel is well established in aesthetic facial procedures especially on the nasolabial fold (NLF).
Objective
To compare the efficacy and safety of PP-501-A-Lidocaine dermal filler with RestylaneLidocaine® when administered to the NLF.
Methods
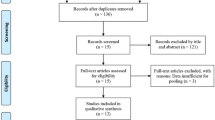
Sixty-six subjects seeking correction of NLFs, with moderate or severe wrinkle severity, were recruited for this multicenter, randomized, patient and evaluator-blind, matched pairs, and active-controlled design clinical study. PP-501-A-Lidocaine and RestylaneLidocaine® were injected into the deep layer of the dermis and/or subcutis of the NLF. The first validity evaluation variable was the average wrinkle severity rating scale (WSRS), as scored by independent blinded evaluators at week 24. The second validity evaluation variable including the global aesthetic improvement scale (GAIS), the WSRS, and adverse event reporting at weeks 8, 16, and 24 were also performed.
Results
The mean improvement in the WSRS from baseline was 1.58 ± 0.68 for the PP-501-A-Lidocaine and 1.51 ± 0.66 for the RestylaneLidocaine® at week 24. The average value at week 8 after the final application was 1.62 ± 0.78 and 1.60 ± 0.75 in parts subject to PP-501-A-Lidocaine and RestylaneLidocaine®, respectively, and 1.58 ± 0.70 and 1.57 ± 0.68 at week 16, respectively. Both improvement and duration of the treatment effect were similar between the two groups. GAIS data rated by the treating investigator and participants showed no statistically significant differences. Both fillers were well tolerated and adverse reactions were mild and transient in most cases.
Conclusion
PP-501-A-Lidocaine showed an equivalent efficacy and safety observed after 6 months of follow-up compared to RestylaneLidocaine®.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to Table of Contents or the online Instructions to Authors www.springer.com/00266.

Similar content being viewed by others
References
Levy PM, Boulle KD, Raspaldo H (2009) A split-face comparison of a new hyaluronic acid facial filler containing pre-incorporated lidocaine versus a standard hyaluronic acid facial filler in the treatment of naso-labial folds. J Cosmet Laser Ther 11:169–173
Beasley KL, Weiss MA, Weiss RA (2009) Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg 25:86–94
Kim JE, Sykes JM (2011) Hyaluronic acid fillers: history and overview. Facial Plast Surg 27:523–528
Narins RS, Brandt F, Leyden J, Leyden J, Lorence ZP, Rubin M, Smith S (2003) A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg 29:588–595
Bentkover SH (2009) The biology of facial fillers. Facial Plast Surg 25:73–85
Beer K (2007) A randomized, evaluator-blinded comparison of efficacy of hyaluronic acid gel and avian-sourced hylan B plus gel for correction of nasolabial folds. Dermatol Surg 33:928–936
Cohen JL, Dayan SH, Brandt FS, Nelson DB, Axford-Gately RA, Theisen MJ (2013) Systematic review of clinical trials of small- and large-gel-particle hyaluronic acid injectable fillers for aesthetic soft tissue augmentation. Dermatol Surg 39:205–231
Monheit GD, Campbell RM, Neugent H, Nelson CP, Prather CL, Bachtell N, Eng D, Holmdahl L (2010) Reduced pain with use of proprietary hyaluronic acid with lidocaine for correction of nasolabial folds: a patient blinded, prospective, randomized controlled trial. Dermatol Surg 36:94–100
Brandt FS, Cazzaniga A (2008) Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging 3:153–159
Rao J, Chi GC, Goldman MP (2005) Clinical comparison between two hyaluronic acid–derived fillers in the treatment of nasolabial folds: hylaform versus restylane. Dermatol Surg 31:1587–1590
Friedman P, Mafong E, Kauvar A, Geronemus R (2002) Safety data of injectable non-animal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg 28:491–494
Jones DTA, Borrell M (2010) In vitro resistance to degradation of hyaluronic aciddermal fillers by ovine testicular hyaluronidase. Dermatol Surg 36:804–809
Smith KC (2007) Practical use of Juvéderm: early experience. Plast Reconstr Surg 120(Suppl):67–73
Narins RS, Dayan SH, Brandt FS, Baldwin EK (2008) Persistence and improvement of nasolabial fold correction with non animal stabilized hyaluronic acid 100,000 gel particles/mL filler on two retreatment schedules: results up to 18 months on two retreatment schedules. Dermatol Surg 34:S2–S8
Narins RS, Brandt FS, Dayan SH, Hornfeldt CS (2011) Persistence of nasolabial fold correction with a hyaluronic acid dermal filler with retreatment: results of an 18-month extension study. Dermatol Surg 37:644–650
Borrell M, Leslie DB, Tezel A (2011) Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther 13:21–27
Lowe NJ, Maxwell CA, Patnaik R (2005) Adverse reactions to dermal fillers: review. Dermatol Surg 31:1616–1625
Weinkle SH, Bank DE, Boyd CM, Gold MH, Thomas JA, Murphy DK (2009) A multi-center, double-blind, randomized controlled study of the safety and effectiveness of Juve´derm injectable gel with and without lidocaine. J Cosmet Laser Ther 8:205–210
Acknowledgments
This study was sponsored by Pacific pharma, who provided the PP-501-A-Lidocaine and Restylanelidocaine® products.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest, real, or perceived.
Rights and permissions
About this article
Cite this article
Choi, W.J., Han, S.W., Kim, J.E. et al. The Efficacy and Safety of Lidocaine-Containing Hyaluronic Acid Dermal Filler for Treatment of Nasolabial Folds: A Multicenter, Randomized Clinical Study. Aesth Plast Surg 39, 953–962 (2015). https://doi.org/10.1007/s00266-015-0571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-015-0571-z