Abstract
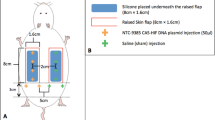
Use of a skin flap has been a common technique in reconstructive surgery for more than five decades. However, partial necrosis of its distal end is still a serious postoperative complication. Many theories about this problem have been proposed, including deficient blood supply, which is the most accepted theory. In this study we demonstrated that hypoxic preconditioning enhanced the viability of adipose-derived stem cells (ADSCs) in vivo and improved their ability to increase the survival rate of ischemic skin flaps in rats. Seven days after flap elevation, the flap survival rate in the hypoxic preconditioned ADSC group was higher than that in the control group. Moreover, histological examination showed that more ADSCs survived in flaps treated by hypoxic preconditioning. Vascular density in the hypoxic preconditioned ADSC group was 30–90 % greater than that in the control group. In addition, the expressions of vascular endothelial growth factor and hypoxia inducible factor-1α (HIF-1α) were higher in the hypoxic preconditioned ADSC group than in the control group (p < 0.05). This enhancive phenomenon reached its highest level at the precondition times of 3 and 7 days in the hypoxic preconditioned ADSC group. We conclude that hypoxia preconditioning effectively enhances the viability of ADSCs to increase the survival rate of ischemic skin flaps. Furthermore, 3 days is the optimal preconditioning time point.
Level of Evidence II
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.









Similar content being viewed by others
References
Tepper OM et al (2005) Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105(3):1068–1077
Park S et al (2004) Selective recruitment of endothelial progenitor cells to ischemic tissues with increased neovascularization. Plast Reconstr Surg 113(1):284–293
Ceradini DJ et al (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10(8):858–864
Morris SF, Taylor GI (1995) The time sequence of the delay phenomenon: when is a surgical delay effective? An experimental study. Plast Reconstr Surg 95(3):526–533
O’Toole G et al (2002) Vascular endothelial growth factor gene therapy in ischaemic rat skin flaps. Br J Plast Surg 55(1):55–58
Uhl E et al (1994) Improvement of skin flap perfusion by subdermal injection of recombinant human basic fibroblast growth factor. Ann Plast Surg 32(4):361–365 discussion 365–366
Uysal AC et al (2009) The effect of adipose-derived stem cells on ischemia-reperfusion injury: immunohistochemical and ultrastructural evaluation. Plast Reconstr Surg 124(3):804–815
Simman R, Craft C, McKinney B (2005) Improved survival of ischemic random skin flaps through the use of bone marrow nonhematopoietic stem cells and angiogenic growth factors. Ann Plast Surg 54(5):546–552
Lee EY et al (2009) Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17(4):540–547
Rey S, Semenza GL (2010) Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res 86(2):236–242
Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL (2003) Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93(11):1074–1081
Elson DA et al (2000) Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res 60(21):6189–6195
Li W et al (2007) Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J 26(5):1221–1233
Yang D, Morris SF (1998) Comparison of two different delay procedures in a rat skin flap model. Plast Reconstr Surg 102(5):1591–1597
Nie C et al (2011) Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 20(2):205–216
Gimble JM, Guilak F (2003) Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol 58:137–160
Nakagami H et al (2006) Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb 13(2):77–81
Izadpanah R et al (2006) Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 99(5):1285–1297
Zuk PA et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Rehman J et al (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109(10):1292–1298
Reichenberger MA et al (2012) Adipose derived stem cells protect skin flaps against ischemia-reperfusion injury. Stem Cell Rev 8(3):854–862
Giatromanolaki A et al (2010) Hypoxia and activated VEGF/receptor pathway in multiple myeloma. Anticancer Res 30(7):2831–2836
Lee S et al (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130(4):691–703
Gao W et al (2011) Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1alpha. J Cell Mol Med 15(12):2575–2585
Lu F et al (2008) Improved viability of random pattern skin flaps through the use of adipose-derived stem cells. Plast Reconstr Surg 121(1):50–58
Stubbs SL et al (2012) Hypoxic preconditioning enhances survival of human adipose-derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev 21(11):1887–1896
Ghali S et al (2007) Vascular delay revisited. Plast Reconstr Surg 119(6):1735–1744
Acknowledgments
This research project was supported by National Natural Science Foundation of China Grants 81171825
Conflicts of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peipei Zhang, Yeli Yue, and Dan Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yue, Y., Zhang, P., Liu, D. et al. Hypoxia Preconditioning Enhances the Viability of ADSCs to Increase the Survival Rate of Ischemic Skin Flaps in Rats. Aesth Plast Surg 37, 159–170 (2013). https://doi.org/10.1007/s00266-012-9993-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-012-9993-z