Abstract
Background
Phosphatidylcholine formulation has been used to dissolve local fat deposits. This study aimed to evaluate and compare the effects of phosphatidylcholine formulation and its vehicle sodium deoxycholate alone on different cell lines to understand better its mechanism of action.
Methods
Cells and media including 3T3-L1 preadipocytes, normal foreskin fibroblasts, neonatal human dermal microvascular endothelial cells (CADMEC), and fetal human skeletal muscle cells (HSkMC) were used.
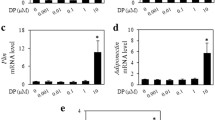
After 24 h, cells were exposed in 3-4, 5-dimethylthiazol-2-yl-2, 3-diphenyl tetrazolium bromide reagent (MTT assays) to increasing dosages of phosphatidylcholine formulation (0.0156–0.5 mg/ml) or an equivalent vehicle, sodium deoxycholate solution, pH 9.0 (0.0066–0.210 mg/ml). Viability was assessed after 1, 2, and 3 days of treatment. Fat tissue (4 × 4 cm) obtained ex vivo from the dorsal fat pads of five rabbits was injected with 2 ml of phosphatidylcholine formulation (50 mg/ml), sodium deoxycholate (21 mg/ml), or normal saline and incubated for 24 h. These were examined histologically to identify cell lysis and morphologic changes.
Results
At 0.125- and 0.25-mg/ml doses of phosphatidylcholine solution, CADMEC and HSkMC were more sensitive (P < 0.001, one-way ANOVA) than adipocytes at all time points examined. Phosphatidylcholine formulation at a dose of 0.5 mg/ml and the equivalent vehicle, sodium deoxycholate, at a dose of 0.21-mg/ml both induced nearly 100% fat cell lysis after 24 h, and evidence of cell lysis as early as 6 h after exposure. After incubation of fat tissue for 24 h with phosphatidylcholine formulation, loss of intracellular lipid staining with an increase in extracellular lipids was seen.
Conclusions
Isolated sodium deoxycholate was almost as effective as the phosphatidylcholine formulation, at clinical concentrations, in reducing the viability of mature adipocytes over time. Similar cytotoxic effects of phosphatidylcholine formulation on normal foreskin fibroblasts, endothelial cells, and human skeletal muscle cells also were observed. The data prove that the formulation acts in a nonspecific manner and that its unintentional administration to other tissues causes cell death.






Similar content being viewed by others
References
Pistor M (1976) What is mesotherapy? Chir Dent Fr 46:59–60
De Beir J, Bazon H (1984) On the subject of mesotherapy. Chir Dent Fr 54:27–28
Gallo R (1980) Mesotherapy in phlebology. Phlebologie 33:153–156
Menkes CJ, Laoussadi S, Kac-Ohana N, Lasserre O (1990) Controlled trial of injectable diclofenac in mesotherapy for the treatment of tendonitis. Rev Rhum Mal Osteoartic 57:589–591
Hexsel D, Serra M, Mazzuco R, Dal’Forno T, Zechmeister D (2003) Phosphatidylcholine in the treatment of localized fat. J Drugs Dermatol 2:511–518
Young VL (2003) Lipostabil: the effect of phosphotidylcholine on subcutaneous fat. Aesthet Surg J 23:413–417
Bauman LS (2003) Phosphatidylcholine. Skin Allergy News 34:24
Nattermann International GMBH (1990) Lipostabil. Nattermann International, Cologne, Germany, p 9
Maggiori S (1988) Treatment of xanthelasma with phosphatidylcholine. Fifth international meeting of mesotherapy, Paris, France
Rittes PG (2001) The use of phosphatidylcholiefor correction of lower lid bulging due to prominent fat pads. Dermatol Surg 27:391–392
Rittes PG (2003) The use of phosphatidylcholine for correction of localized fat deposits. Aesth Plast Surg 27:315–318
Duncan DI, Hasengschwandtner F (2005) Lipodissolve for subcutaneous fat reduction and skin retraction. Aesth Surg J 25:530–543
Ablon G, Rotunda AM (2004) Treatment of lower eyelid fat pads using phosphatidylcholine: clinical trial and review. Dermatol Surg 30:422–427
Rotunda AM, Suzuki H, Moy RL, Kolodney MS (2004) Detergent effects of sodium deoxycholate are a major feature of an injectable phospatidylcholine formulation used for localized fat dissolution. Dermatol Surg 30:1001–1008
Moy LS (2004) Phosphatidylcholine Injections. A study measuring decreased subcutaneous fat thickness. American Society for Dermatologic Surgery and the American Society of Mohs Micrographic Surgery and Cutaneous Oncology combined annual meeting, San Diego, CA, 30 Sept–3 Oct 2004
Ablon G (2005) Preliminary experience with mesotherapy utilizing phosphatidylcholine. American Society for Dermatologic Surgery-American College of Mohs Micrographic Surgery and Cutaneous Oncology combined annual meeting, Atlanta, GA, 27–30 Oct 2005
Asaadi M, Salas AP, Motamedi B (2004) Mesoplasty: a new approach to nonsurgical liposculpture. American Society of Plastic Surgery. Philadelphia, PA, 9 Oct 2004
Rose PT, Morgan M (2005) Histological changes associated with mesotherapy for fat dissolution. J Cosmet Laser Ther 7:17–19
Bechara FG, Sand M, Sand D et al. (2005) Ultrasound-controlled injection lipolysis of lipomas with phosphatidylcholine in patients with familial multiple lipomatosis. American Society for Dermatologic Surgery-American College of Mohs Micrographic Surgery and Cutaneous Oncology combined annual meeting, Atlanta, GA, 27–30 Oct 2005
Rullan P, Hexsel D (2005) Phosphatidylcholine injections for lipolysis of neck and jowls: 50 case presentation. American Society for Dermatologic Surgery-American College of Mohs Micrographic Surgery and Cutaneous Oncology combined annual meeting, Atlanta, GA, 27–30 Oct 2005
American Society for Aesthetic Plastic Surgery (October 2002) Lipoplasty (liposuction) without surgery? The American Society for Aesthetic Plastic Surgery, New York. Retrieved at http://www.surgery.org/press/news-print.php?iid=208§ion=news-lipoplasty
Rotunda AM, Kolodney MS (2006) Mesotherapy and phosphatidylcholine infections: Historical clarification and review. Dermatol Surg 32:465–480
Linchtenberg D, Robertson RJ, Dennis EA (1983) Solubilization of phospholipids by detergents: Structural and kinetic aspects. Biocbhimcal et Biophysica Acta 737:285–304
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, A., Lobocki, C., Singh, S. et al. Actions and Comparative Efficacy of Phosphatidylcholine Formulation and Isolated Sodium Deoxycholate for Different Cell Types. Aesth Plast Surg 33, 346–352 (2009). https://doi.org/10.1007/s00266-008-9301-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-008-9301-0