Abstract
Background
This clinical and experimental study compared adipose tissue transplant behavior after two different techniques of purifying: centrifugation at 3400 rpm for 3 min and serum lavage without centrifugation.
Methods
Clinical evaluation was performed under standardized conditions for lipofilling on a series of 51 female patients, intentionally selected to have similar characteristics and assigned to two groups based on the method of processing. Experimentally, a culture system in diffusion chambers with vitaline membranes was designed to mimic the behavior and to study the morphology of the adipose tissue used for autografting. Survival, structure, and proliferation of the adipose cells in vitro were examined by classical histologic H&E staining and immunohistochemistry for leptin and cyclin D1.
Results
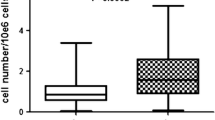
The main differences encountered experimentally were the presence of a greater amount of preadipocytes in the noncentrifuged adipose tissue cultures and more distinctly expressed cell proliferation. The postoperative clinical results favored of the serum lavage purifying technique.
Conclusion
Our data suggest that with transplantation of noncentrifuged adipose tissue more active preadipocytes are applied which could possibly lead to better potential chances of survival and even de novo development of fat.



Similar content being viewed by others
References
Neuber GA (1893) Fettransplantation. Chir Kongr Verhandl Dtsch Gesellsch Chir 22:66
Illouz YG (1986) The fat cell graft: a new technique to fill depressions. Plast Reconstr Surg 78:122–123
Coleman SR (1994) The technique of periorbital lipoinfiltration: operative techniques. Plast Reconstr Surg 1:120–134
Coleman SR (1995) Long term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 19:421–425
Billings E Jr, May JW Jr (1989) Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg 83:368–381
Mojallal A, Foyatier JL (2004) The effect of different factors on the survival of transplanted adipocytes. Ann Chir Plast Esthét 49:426–436; in French
Chajhir A, Benzaquen I, Moretti E (1993) Comparative experimental study of autologous adipose tissue processing by different techniques. Aesthetic Plast Surg 17:113–115
Niechajev I, Sevcuk O (1994) Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg 94:496–506
Shiffman MA, Mirrafati S (2001) Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg 27:819–826
Mojallal A, Breton P, Delay E, Foyatier J-L (2004) Greffe d’adipocytes: applications en chirurgie plastique et esthetique. Encyclopedie Medico-Chirurgicale: Techniques chirurgicales, 1st edn. Elsevier SAS, Amsterdam
Coleman SR (2001) Structural fat grafts: the ideal filler? Clin Plast Surg 28:111–119
Jauffret JL, Champsaur P, Robaglia-Schlupp A, Andrac-Meyer L, Magalon G (2001) Arguments in favor of adipocyte grafts with the S.R. Coleman technique. Ann Chir Plast Esthét 46:31–38; in French
Lalikos JF, Li YQ, Roth TP, Doyle JW, Matory WE, Lawrence WT (1997) Biochemical assessment of cellular damage after adipocyte harvest. J Surg Res 70:95–100
Moore JH Jr, Kolaczynski JW, Morales LM, Considine RV, Pietrzkowski Z, Noto PF (1995) Viability of fat obtained by syringe suction lipectomy: effects of local anesthesia with lidocaine. Aesthetic Plast Surg 19:335–339
Novaes F, Reis N, Baroudi R (1998) Counting method of live fat cells used in lipoinjection procedures. Aesthetic Plast Surg 22:12–15
MacRae JW, Tholpady SS, Katz AJ, Gampper TG, Drake DB, Ogle RC, Morgan RF (2003) Human adipocyte viability testing: a new assay. Aesthetic Surg J 23:265–269
Tavassoli M (1982) In vivo development of adipose tissue following implantation of lipid-depleted cultured adipocyte. Exp Cell Res 137:55
Engenmann G, Hauner H (1996) Relationship between replication and differentiation cultured human adipocyte precursor cells. Am J Physiol 270:C1011
Schoeller T, Lille S, Wechselberger G, Otto A, Mowlawi A, Piza-Katzer H (2001) Histomorphologic and volumetric analysis of implanted autologous preadipocyte cultures suspended in fibrin glue: a potential new source for tissue augmentation. Aesthetic Plast Surg 25:57–63
Georgiev I (1973) Cultures organotypiques in vitro en chambres a diffusion. I-e Congres de Societe des Pathologistes bulgares, Sofia, Bulgaria, 1973
Atanassova P, Popova E (2000) Leptin expression during the differentiation of subcutaneous adipose cells of human embryos in situ. Cells Tissues Organs 166:15–19
Yin JH, Li M, Yang J, Wu CY (2007) Primary culture of human omental preadipocytes and study of their biological properties. Zhonghua Yi Xue Za Zhi 12:838–841
Hudson DA, Lambert EV, Bloch CE (1990) Site selection for fat autotransplantation: some observation. Aesthetic Plast Surg 14:195–197
von Heimburg D, Hemmrich K, Zachariah S, Staiger H, Pallua N (2005) Oxygen consumption in undifferentiated versus differentiated adipogenic mesenchymal precursor cells. Respir Physiol Neurobiol 146:107–116
Peer LA (1956) The neglected free fat graft. Plast Reconstr Surg 18:234–250
Kirkland JL, Hollenberg CH, Gillon WS (1990) Age, anatomic site and the replication and differentiation of adipocyte precursors. Am J Physiol 258:206–210
Butterwith SC (1994) Molecular events in adipocyte development. Pharmacol Ther 61:399–411
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khater, R., Atanassova, P., Anastassov, Y. et al. Clinical and Experimental Study of Autologous Fat Grafting After Processing by Centrifugation and Serum Lavage. Aesth Plast Surg 33, 37–43 (2009). https://doi.org/10.1007/s00266-008-9269-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-008-9269-9