Abstract
In species where conflict is costly, individuals adopt alternative movement tactics to minimise the risk of competitive interactions. Dominant males often maintain defined territories, while less competitive males may be forced to adopt alternative tactics to maximise fitness and reduce conflict. However, the extent to which males switch tactics according to current social or physiological status is poorly understood. Using implanted acoustic tags and a fixed array of tracking receivers, we investigated how the behaviour of 78 male estuarine crocodiles (Crocodylus porosus) shifted over an 11-year period in relation to ontogeny, body condition, and the extent of physical injuries. We discovered that male crocodiles sorted into three common movement classes, with 51% of males maintaining the same movement class across consecutive years (max = 9 years). Males > 4 m in total length maintained confined territories both within and across years and had the greatest extent of injuries and the highest condition score, indicative of territory holders. In contrast, smaller males sorted into high movement roamer or low movement site-philopatric tactics, where the tactic an individual adopted was less stable between years and did not correlate with condition or external injuries. Our study reveals the socio-biological mechanisms by which estuarine crocodiles coexist within a restricted habitat.
Significance statement
Identifying individual-level differences in movement helps us predict which individuals are more likely to be involved in human-wildlife interactions. However, studying long-term shifts in movement is challenging, as large datasets of co-occurring individuals tracked in their natural environment over multiple years are required. We tracked a population of 78 male estuarine crocodiles (1030–4687 mm total length) in a shared environment over 11 years and assessed how eight movement traits were linked to body size and physical condition. At the population level, males sorted into different movement tactics according to ontogeny, with large territorial males having better body condition yet a greater incidence of injury. However, 49% of males showed variability across years, suggesting that tactics were conditional relative to environmental variability and a male’s own status. Our study provides insights into the mechanisms and costs of movement tactics in wild crocodile populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Movement is a constant across the life stages of most organisms (Nathan et al. 2008). From the ocean-scale migrations of cetaceans (Dalla Rosa et al. 2008) to the deliberate fine-scale movements observed in self-propelled microorganisms (Clement 1987), moving for survival and reproduction is an almost defining characteristic of all animal species. Technological advances have also revealed that individuals of the same species are not homogenous in the way they behave and use space (Campbell et al. 2013; Smetzer et al. 2021; Stuber et al. 2022). For example, individuals from different populations may vary in their migratory tendency, with some populations adopting a migratory strategy while others are more sedentary (Newton 2008). Individuals among the same population also often show striking intraspecific variation in movement patterns and foraging behaviour (Baird et al. 2012; Debeffe et al. 2012). Movement decisions can be driven by external factors such as differences in an individual’s social (Wolf and Trillmich 2007) and physical environment (Fujisaki et al. 2014). Alternatively, these decisions can be driven by an individual’s internal state (Nathan et al. 2008; e.g. motivation to find a mate: Taborsky et al. 2008; hunger level: Spiegel et al. 2015; or age, size, or physical condition: Martin et al. 2013; Melzheimer et al. 2018; Horváth et al. 2020).
An individual’s tendency to move or to stay put is determined by the costs and benefits of the movement given their internal state (Michelangeli et al. 2022). By specialising in their movement tactic, individuals can reduce competition over resources and so reduce the costs of social conflict (Gross 1996; Taborsky et al. 2008; Schradin et al. 2009; Rimbach et al. 2019). For example, adult males may fight to maintain exclusive territories (Leboeuf 1974; McElligott et al. 1998), where territorial disputes can result in high physiological costs (Schradin et al. 2009) and an elevated risk of physical injury (Baird et al. 2012). Males which lack the ability to defend a territory will often resort to alternative movement tactics to reduce agonistic interactions (Baird et al. 2012; Melzheimer et al. 2018). However, individuals following these alternative movement tactics may experience reduced access to resources (Baldi et al. 1996; Melzheimer et al. 2018). As such, these tactics are “conditional” in the way they depend on an individual’s assessment of the tactic’s relative benefits given their current physical and physiological status (Clutton-Brock 1989).
Identifying the causes, patterns, mechanisms, and consequences of such individual variation in movement traits has been a key topic in ecological research (Nathan et al. 2008). However, it has been hindered by the problems inherent in inferring natural behaviours in experimental studies using captive animals, or challenges observing the movements of individuals throughout their natural environments over extended periods. Hertel et al. (2020) identified three requirements to the study of individual behavioural differences from animal tracking data: 1) that the temporal resolution of the movement data was adequate to detect the focal behaviour, 2) that monitoring durations were long enough to obtain a sufficient number of “repeats” of movement behaviours (i.e. across multiple consecutive years), and 3) that a sufficient sample of individuals is tracked from the same population to statistically estimate individual-level patterns (Hertel et al. 2020). Advances in acoustic telemetry technology that uses fixed-position acoustic receivers and uniquely coded acoustic transmitters are providing new insights into how animals move and interact within their natural environment (Hussey et al. 2015). Not only does this technology facilitate large numbers of individuals within the same population to be tracked concurrently but also a high transmission rate (< 120 s) and the extended battery life of acoustic transmitters (up to 10 years) allow individuals to be tracked at high resolution for a large proportion of their lifetime.
In this study, we used acoustic telemetry to investigate the presence of alternative movement tactics in a population of male estuarine crocodiles (Crocodylus porosus). Estuarine crocodiles are sexually dimorphic (Webb and Messel 1978), with males growing up to ~ 40% larger than females (max size = ~ 6 m, Britton et al. 2012) and individuals continuing to grow throughout much of their adult lives (Baker et al. 2019). Competition among males for access to reproductive females is believed to be the driving force behind the evolution of this sexual dimorphism (Webb and Smith 1987). Only the largest, most competitive males are known to aggressively defend established territories against conspecifics (Lang 1987), while intermediate-sized males roam more widely and occupy larger home ranges (Campbell et al. 2013; Hanson et al. 2015). Together, this suggests that male estuarine crocodiles exhibit diverging behaviour tactics, with smaller males adopting a high movement tactic until their competitive ability increases and they can switch to a low movement tactic to invest in the defence of established territories. However, both Hanson et al. (2015) and Campbell et al. (2015) revealed exceptions to this general trend where some smaller male crocodiles chose to remain within a confined area while other very large males (> 4 m total body length) roamed over extensive areas. Due to the short timeframe of these studies (6–24 months), the consistency of movement behaviours across multiple years was not investigated. Similarly, a small sample size and a lack of any condition metrics (other than body length) meant the role of an individual’s internal state in driving individuals to follow different movement tactics was not assessed. Here, we tracked the movement behaviours of 78 tagged male crocodiles across 11 consecutive years to examine: (1) if males could be assigned to different movement tactics according to movement patterns, (2) if our classification of crocodile movement tactics were consistent within individuals across multiple consecutive years, and (3) if males following different movement tactics differ in their body size, the extent and location of injuries, and their overall body condition.
Methods
Study site and crocodile capture
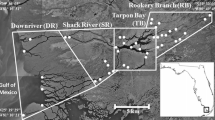
Between 2008 and 2020, up to 20 crocodile traps were deployed along a 47-km stretch of the Wenlock River, Cape York Peninsula, Australia (Fig. 1). Traps were set between August and September each year, and either floated on the water surface or were placed at the high-tide mark along the riverbank. Traps were baited with wild pig (Sus scrofa) or cow (Bos taurus), with the trap door sprung by a trigger mechanism attached to the bait. For individuals < 2 m in total length, hand capture via spotlighting with a noose was also used. Once crocodiles were restrained, records were taken of sex, total body length (TL), snout-vent length (SVL) measured to the posterior margin of the cloaca, and tail girth (TG) measured around the same point, and transmitters implanted before individuals were released at their point of capture. During capture, crocodiles were also examined for signs of injury including puncture wounds, missing limbs, scars, or split scales. As the tail of many captured crocodiles was damaged upon inspection, SVL was chosen over total length as a more representative index of crocodile body size (Supplementary Fig. 1).
Remote monitoring of crocodile movements
Coded acoustic transmitters (V13T or V16T, www.innovasea.com) were implanted into crocodiles following Franklin et al. (2009). In brief, a local anaesthetic (lignocaine) was injected subcutaneously behind the left forelimb. Following aseptic technique, a small pocket was then formed under the skin with blunt-ended scissors, into which the acoustic transmitter was inserted. The incision was closed using monofilament sutures and sprayed with antibiotic.
To detect the implanted acoustic transmitters, an array of underwater acoustic receivers (VR2-W, Innovasea) was deployed throughout the Wenlock River for the duration of the study (Fig. 1a). Acoustic receivers were spaced approximately 1–5 km apart and were attached to concrete anchors positioned approximately 1– 2 m below the water surface and 2–20 m from the riverbank. Each receiver had a detection radius of approximately 400 m, and as the river width was typically less than 100 m and pulse transmission rate of the acoustic tags was set randomly between 90 and 120 s, it was unlikely that crocodiles could pass by a receiver without being detected.
Our data was not recorded blind, as we were studying focal animals in a field setting.
Calculation of movement metrics
To classify the movement behaviour adopted by tagged male crocodiles, eight movement metrics were calculated for each individual for each month it was detected on the acoustic array. To examine the prevalence of acoustic tagged crocodiles both inside and outside the study area, we examined the frequency of detection of each acoustic-tagged crocodile on three spatial scales. On a broad scale, we calculated the proportion of time a tagged crocodile spent within the boundary of our study area for a given month (presence); on a medium scale, we used the proportion of days an individual was detected on acoustic receivers while within the boundaries of the study area (days detected); on a fine scale, we used the number of times an individual was detected by a receiver per day (centres of activity per day). Further detail on the steps to generate these metrics can be found in the Supplementary Materials.
To describe the extent of areas utilised by tagged crocodiles, we generated monthly home ranges for each individual using least cost utilisation distributions (lcUD) following the methods described in Dwyer et al. (2020) and Baker et al. (2021, Supplementary Materials). Core (50% lcUD) and extent home ranges (95% lcUD) were generated for each individual for each month, with lcUD calculations based on 6-h centres of activity (COAs). In addition, we also calculated the spatial overlap of 95% lcUDs with the home range extent of the previous month to provide a measure of site philopatry (i.e. how consistent home ranges were between tracking months). Males had to be detected at least five times per month at three unique COA positions to generate home range estimates. Any individual who did not meet this threshold for at least 1 month was excluded from the study.
To quantify movements within an animal’s home range, we generated two movement metrics that aimed to differentiate between wide-ranging travel and patrolling behaviour (Supplementary Materials). First, we used the mean maximum distances a tagged crocodile travelled per day based on 6-h COA locations, with COAs chosen over raw detections to prevent overinflated estimates of distances travelled due to overlapping receiver detection fields. Next, we determined the average distance between each COA and the edge of their 95% monthly home range (lcUD) centroid in order to differentiate between wide-ranging (large distances) and patrolling behaviour (low distances) (Campbell et al. 2013).
Assigning behavioural movement class
To assign each of our acoustic tagged male crocodiles to a class of movement behaviour, we performed a principal components analysis (PCA) across the eight movement metrics using the FactoMineR package in R (Le et al. 2008). All monthly movement metrics were averaged over an individual’s entire detection period and then standardised prior to the PCA analysis. Ward’s agglomerative hierarchical clustering was then applied to the first two principal components to determine the optimal number of behavioural movement classes present. The tree produced by hierarchical clustering was cut based on the inertia gain criterion (Argüelles et al. 2014). Finally, to produce more robust clusters, K-means clustering was performed on the data based on the number and gravity centres of clusters obtained.
To investigate how individuals in each of our defined movement classes differed in their movement behaviour, we constructed a series of general (or generalised) linear models (GLMs) with a single record for each tagged crocodile. In these models, the movement metric of interest was used as the response variable and the cluster id (i.e. the behavioural movement class) as the predictor variable. Most GLMs assumed a Gaussian distribution; however, a binomial GLM was used to test for between-class differences in the number of days tagged crocodiles were present in the study area vs. the number of days they were absent. If a significant effect was found (p < 0.05), differences between clusters were further investigated using Tukey tests.
Annual variability in movement class assignment
To assess whether tagged male crocodiles shifted behavioural movement class between tracking years, separate PCAs and cluster analyses were performed for each male for each year they were tracked. In these analyses, a single year of tracking data was extracted for a single male while all other males in the analysis remained constant (i.e. with their monthly metrics averaged over their entire detection period). The number of clusters was also kept consistent with those adopted in the original analysis. This allowed us to obtain yearly cluster alignments for each male while maintaining the integrity of the original cluster analysis.
Quantifying body condition and injuries
To examine how the body condition of male crocodiles varied according to behavioural movement class, we calculated for each male a condition score following Nilsen et al. (2017):
where C is body condition, TG is tail girth (mm), and SVL is snout vent length (mm). We generated scores for each individual per capture year (to account for recaptures) based on the SVL and TG measurements to identify any annual variation in body condition. A linear mixed-effects model (LME) was then constructed using the lme4 R package (Bates et al. 2015), with body condition as the response variable and SVL, movement class, and the two-way interaction between SVL and movement class as fixed effects. Individual ID was included as a random effect to account for repeated measurements from recaptured individuals.
To examine how the probability of injury varied according to body size and behavioural movement class, injuries to the head, body, limbs, or tail locations were recorded as either present or absent for each year a crocodile was captured. Where possible, only injuries deemed to be recent were included in the analysis to reduce bias towards larger (and older) crocodiles. We then constructed a series of generalised linear mixed-effects models (GLMM) with a binomial distribution (logit link) for each injury location, with injury presence (or absence) as the response variable, SVL, movement class, and the two-way interaction between SVL and movement class as predictor variables, and individual ID as a random effect.
Results
Between 2008 and 2020, 119 male estuarine crocodiles (272–2510 mm SVL, 563–4687 mm total length) were captured on the Wenlock River and implanted with acoustic transmitters. Following release, crocodiles were tracked via the acoustic receiver array for periods ranging between 30 and 4100 days (mean = 1443 days; Supplementary Fig. 2), with between 400 and 340,000 tag detections recorded per crocodile. Of these, 78 individuals (509–2510 mm SVL; Supplementary Table 1) possessed the minimum required data for inclusion within the study (i.e. a minimum of three unique centres of activity per month for > 1 month).
PCA
The first two principal components explained 77.1% of the variability observed in our eight movement metrics and were retained for subsequent analyses. The first principal component (PC1) explained 51.9% of the variation, with positive loadings for core home range area (km2), extent home range area (km2), distance per day (km.day−1), the mean monthly distance a crocodile moved from their home range centroid (km), and a negative loading of the proportion of time present within the upstream and downstream extent of our study area (Fig. 2). The second principal component (PC2) explained 25.3% of the variability in our movement metrics, with days detected per month, the number of COA locations per day, and monthly home range overlap all having positive loadings (Fig. 2). In terms of crocodile behaviour, PC1 describes the area of river crocodiles occupied and the distance travelled within their home range. In contrast, PC2 describes how frequently a crocodile was detected while within the boundaries of the study area and the similarity between monthly home ranges (i.e. a crocodile’s degree of site philopatry).
Movement classes of acoustic-tagged male estuarine crocodiles (Crocodylus porosus), visualised using the first two principal components produced from eight movement metrics (presence: proportion of time present in the study area, days detected: proportion of days detected while in the study area, COAs per day; 6-hourly centres of activity per day, overlap; percent overlap between successive monthly home ranges, distance per day: mean distance travelled each day, distance from centroid: mean distance of COAs from monthly home range centroid, core HR: area of 50% lcUD, and extent HR: area of 95% lcUD. Each dot represents the entire tracking period of one individual. Arrows show the influence of each movement metric such that PC 1 represents activity and PC 2 represents site fidelity, and box plots show the variance of each k-means-derived cluster along the two PCs: boxes indicate the interquartile range (IQR), the central line shows the median, the whiskers extend to 1.5*IQR, and outliers are plotted individually
Behavioural movement classes
Male crocodiles were clustered into three distinct behavioural movement classes based on Ward’s agglomerative hierarchical clustering of first two principal components: class A, class B, and class C (Fig. 2, Supplementary Fig. 3). Class A was characterised by the smallest core (Fig. 3a; GLM, df = 75, T = 2.84, p = 0.006) and extent home ranges (Fig. 3b; GLM, df = 75, T = 2.39, p = 0.019), the lowest daily distances travelled (Fig. 3c; GLM, df = 75, T = 5.73, p < 0.001), and was the cohort that generally remained in closest proximity to their home range centroids (Fig. 3d; GLM, df = 75, T = 3.40, p = 0.001). Class A individuals were also detected on more days per month (Fig. 3f; GLM, df = 75, Z = − 4.34, p < 0.001) and had more consistent home ranges (Fig. 3h; GLM, df = 75, Z = − 6.63, p < 0.001) than individuals in other classes. The core and extent home ranges and amount of movement of class B males fell between those of classes A and C, and these individuals were also detected fewer times per day than both other classes (Fig. 3g; GLM, df =75, T = − 5.964, p < 0.001). Tagged crocodiles in class C were characterised by the largest core (Fig. 3a; > 2.5 km2; GLM, df = 75, T = 10.6, p < 0.001) and extent home ranges (Fig. 3b; GLM, df = 75, T = 9.93, p < 0.001), travelled the greatest distances per day (Fig. 3c; GLM, df = 75, T = 11.9, p < 0.001 ), and generally remained further from their home range centroid (Fig. 3d; GLM, df = 75, T = 13.826, p < 0.001). Class C males also spent longer periods outside the boundaries of our study area than any other movement class (Fig. 3e; GLM, df = 1190, Z = − 32.42, p < 0.001). Finally, class C males held less consistent home ranges between months than class A males (GLM, df = 75, Z = − 4.55, p < 0.001), but their degree of monthly home range overlap was similar to class B males (Fig. 3h).
Of the 78 male estuarine crocodiles which had sufficient detection data, 18 were assigned to movement class A, 45 were assigned to class B, and 15 to class C based on their PC scores for the entire tracking period (Fig. 4a). In our 18 smallest males (< 1400 mm SVL), 61% (n = 11) were assigned to class B and 39% (n = 7) were assigned to class A (Fig. 4a). Of our seven largest males (> 2200 mm SVL), five were assigned to class A, and the remaining two were assigned to class B. No male crocodiles with SVL < 1400 mm or > 2200 mm were assigned to class C for their entire tracking period. Of the 52 males ranging between 1400 and 2200 mm SVL, 62% (n = 32) were assigned to class B, 29% (n = 15) were assigned to class C, and 12% (n = 6) were assigned into class A.
Tiled plot showing the behavioural movement class of male estuarine crocodiles (Crocodylus porosus) for a their entire detection period and b each year of detection. Males are arranged by snout-vent length from the largest males at the top to the smallest at the bottom. * and † differentiate individuals with the same ID
Annual variability in movement class assignment
Of the 63 male crocodiles with more than 1 year of tracking data, 51% (n = 32) were assigned into the same movement class for the duration of their tracking period (Fig. 4b). This included M164b (class A) and M190b (class C) who were tracked for 9 and 7 consecutive years, respectively. Thirteen crocodiles (21%) were assigned into either class A or B in consecutive years, 14 (22%) switched between class B and C, and 4/63 males (6.3%) switched among all three movement classes in consecutive years (Fig. 4b). Small individuals (< 1400 mm SVL, n = 15) displayed relatively stable movement classes between years, with only 22% of individuals switching between classes. Of the seven male crocodiles > 2200 mm in SVL, five (71%) were assigned to class A for ≥ 75% of years tracked (min = 3 years, max = 8 years), while the other two males were assigned to class B on > 60% of years tracked. Four of these males (57%) switched between movement class A and class B in consecutive years. No male crocodiles < 1400 mm SVL or > 2200 mm SVL were assigned to class C for any of the years that they were tracked. Intermediate-sized individuals (1400–2200 mm SVL, n = 41) were more plastic in their movement behaviours between years, with only 41% of individuals with more than 1 year of tracking data assigned to the same class throughout their tracking period. Most intermediate individuals, however, switched between at least two movement classes, with four individuals switching between classes A, B, and C each year during the study.
Relationship between movement class, condition, and external injuries
Of the 78 males assigned to a movement class, 59 had the measurements necessary for estimating body condition and 16 had multiple measurements across years from recapture events. Body condition index in male crocodiles was positively correlated with SVL (LME, F = 13.6, p = 0.001, R2 = 0.46; Fig. 5), where larger males had a higher derived body condition index than smaller males. There was no significant effect of movement class on our index of body condition as either a single effect or as an interaction term in our LME (P > 0.05). Of those recaptured males with multiple estimates of body condition, some individuals exhibited large differences between recapture years, with M243 and M151b showing a marked decline (by 12.5 and 7.7%, respectively) in body condition index across consecutive years (Fig. 5).
Scatter plot showing the body condition index of male estuarine crocodiles (Crocodylus porosus) compared to snout-vent length (mm) in crocodiles in three movement classes. Each point represents a single male, with standard deviation shown for those with multiple measurements across years. Body condition is calculated as \(C=\frac{TG}{\left( SVL\times 2\right)}\) , as marked on the silhouette, where SVL is snout-vent length and TG is tail girth. Linear prediction shows mean ± the 95% confidence interval (LME). Three individuals are highlighted: both M151b and M243 decreased in condition across years, while M220 is the largest class C male and in poor condition for its body size
Of the 68 male estuarine crocodiles that had the presence or absence of external injuries recorded, 10% showed evidence of injuries to the head, 12% had injuries to the body, 16% had injuries to the limbs, and 19% were on the tail. These injuries took the form of gashes, puncture marks from teeth, or missing limbs (Fig. 6). Some individuals presented fresh injuries each year they were captured, with M243 having an increasing number of fresh injuries with each recapture year (i.e. loss of a forelimb and part of a lower jaw), coinciding with a drop in body condition. There was a positive correlation between male body size and the presence of injury on the tail (GLMM, Z = 2.385, p = 0.017) and limbs (GLMM, Z = 2.01, p = 0.045) but not the head (GLMM, Z = 1.78, p = 0.11) or the body (GLMM, Z = 0.767, p = 0.44; Fig. 6). Movement class was not a significant predictor of injury, either as a single term or as an interaction term, in our GLMMs for each injury location (p > 0.05). Male crocodiles had a low (< 0.1) probability of injury to the tail until they passed ~ 1800 mm SVL when the incidence if these injuries markedly increased.
Scatter plot showing the probability of observed external injuries to the body, head, limbs or tail region of male estuarine crocodiles relative to snout vent length. Presence or absence of injury is scored as 1 or 0 respectively, and points jittered in the y axis to facilitate comparison and binomial model predictions for each model (GLM) are shown. Examples of injuries to the body, head, limbs, and tail are shown
Discussion
The existence of alternative movement tactics has been documented across taxa, where individuals in the same population vary in the extent and consistency of their movements. However, due to the challenges of observing large cohorts of individuals continuously in their natural environment, it is often unclear how consistent these tactics are across multiple years or how they relate to an individual’s internal state (i.e. their age, size, or physical condition). By tracking 78 male estuarine crocodiles over an 11-year period, we discovered that individuals segregate into common movement tactics according to how they moved within a shared environment. The tactic that an individual adopted could be partially explained by ontogeny, as the largest males (> 2200 mm SVL) were highly site-attached and maintained confined territories both within and across years, while intermediate-sized (1400–2200 mm SVL) males were more likely to roam and were more plastic in their selection of tactic between years. Fifty-one percent of males tracked for more than 1 year (2–9 years) maintained the same tactic throughout their entire tracking period, while the remaining 49% of individuals switched movement tactics between years. While larger males had better body condition scores, an increasing incidence of injuries to their tail and limbs suggests that there is an inherent cost to defending and/or navigating territories. Our findings suggest that the tactics adopted by male estuarine crocodiles can be highly consistent through time and are conditional according to an individual’s body size but not necessarily their body condition or state of injury.
In polygamous species, the strongest males typically monopolise access to females within defined territories, while smaller and less experienced males are forced to adopt alternative tactics to access mating opportunities (Lincoln 1971; Leboeuf 1974; Clutton-Brock and Albon 1979; Modig 1996; Melzheimer et al. 2018). Estuarine crocodiles are traditionally viewed to live within dominance hierarchies, whereby dominant males control access to females through the expulsion of male conspecifics (Lang 1987). Consistent with this, we found that mature males greater than 2200 mm SVL in our study population maintained discrete home ranges across consecutive months (= class A), while mature individuals between 1400 and 2200 mm SVL were more likely to occupy longer stretches of river and have less site-attachment (class B or C). It is difficult to interpret the movements identified as class B due to the high degree of variation present between individuals within this class. However, the identified movement class C is comparable to a roamer tactic (Schradin et al. 2009; Rimbach et al. 2019), with only sexually mature males less than or equal to 2200 mm SVL adopting this behaviour. In contrast, immature males (< 1500 mm SVL, Webb and Smith 1987) were observed to either display low rates of movement and high site attachment (class A) or moderate home ranges with low site fidelity (class B). This suggests that immature individuals may be adopting a subordinate philopatric tactic (Kingma et al. 2016) where they are tolerated as subordinates by dominant males. Indeed, Baker et al. (2021) found substantial overlaps between male conspecifics across multiple years, suggesting estuarine crocodiles may be more tolerant than previously thought. Research on the association patterns, community composition, and dominance hierarchies of estuarine crocodiles may shed light on the complexities of their social structure.
Alternative movement tactics impart different costs and benefits depending on an individual’s internal state and environment (Clutton-Brock 1989; Strickland et al. 2016). Our study found that larger males had more injuries, with injuries to the head and neck becoming evident upon males only once they attained > 1800 mm SVL and injuries to the tail evident from 1500 mm SVL. However, the largest males, of which most adopted a territorial tactic, also had the highest body condition. This may suggest that the costs of conflict are lower than the benefits of territoriality. Sub-dominant males across taxa may travel over large areas with a low degree of site attachment to limit social conflict with dominant males while maximising their access to reproductive females (Taborsky et al. 2008). However, mature males adopting this roaming tactic in other species have been observed to have higher energetic costs due to greater distances travelled (Rimbach et al. 2019), lower body condition as a result of less consistent access to resources (Melzheimer et al. 2018), reduced growth due to exclusion from feeding opportunities and basking sites (Grigg 2001; Seebacher et al. 2005), and an increased incidence of injury due to more frequent contacts with unfamiliar dominant males (Ridley et al. 2008). The largest class C male (M220) had very low body condition for its size. Despite this, we found that there was no evidence that crocodile physical condition was related to movement tactic, with males having a similar body condition score and incidence of injury to similar-sized conspecifics assigned to other movement classes. Similarly, Strickland et al. (2022) found that body condition, calculated using Fulton’s body condition factor, was a poor predictor of activity in American alligators (Alligator mississippiensis). In this study, we used an estimated measure of body condition that uses tail girth as a proxy for body mass (Nilsen et al. 2017). Further studies examining physiological metrics such as chronic corticosterone as an indicator for stress (Hamilton et al. 2018) and lipid content or fatty acids to assess nutritional state (Meyer et al. 2021) could provide further insights into the influence of physical and physiological status on the selection of movement tactics in male crocodiles.
Mature males across taxa often transition toward territorial movement tactics once they reach a size where the benefits of holding a territory outweigh the costs of defending it (Haley et al. 1994; Wikelski et al. 1996; Melzheimer et al. 2018). Consistent with this, at the population level, we observed a broad association between the movement tactics individuals adopted and ontogeny, as discussed above. However, despite the extended periods over which individuals were tracked (2–9 years), at the individual level, we did not observe any directional shifts in the movement tactic adopted indicative of an inherent transition toward territoriality. Instead, we observed that 51% of males maintained the same movement tactic each year throughout their tracking period, while others shifted tactics between years. Given the long lifespan of estuarine crocodiles (up to 70 years (Webb and Smith 1987)), it is possible that the duration of our study (11 years) was not long enough to observe ontogenetic transitions in behaviour within individuals.
Alternatively, the observed variation may have been due to individual responses to seasonal or inter-annual shifts in their physical and/or social environments (Knapp et al. 2003; Wolf and Trillmich 2007; Baker et al. 2023) rather than changes in ontogeny (Gross 1996). For example, home range size and activity in American alligators are driven primarily by salinity and seasonal changes in hydrology (Rosenblatt and Heithaus 2011; Fujisaki et al. 2014) rather than by body size (Rosenblatt et al. 2013but see Strickland et al. 2022). Female sea lions (Zalophus wollebaeki) have been observed returning to breeding areas with familiar, predictable conspecifics to reduce the potential for conflict (Wolf and Trillmich 2007). Further research is needed to understand how shifts in an individual’s physical and social environments influence the adoption of different movement tactics. Regardless of what is driving the variation in tactics observed in our study, our results highlight the importance of examining both population- and individual-level variations when investigating animal movement and behaviour.
Using a long-term telemetry dataset, we found that male estuarine crocodiles can be separated into three distinct movement classes. Despite being unable to link increases in an individual’s body size to a switch in movement tactic at the individual level, the patterns observed across individuals in this wild population suggest that male estuarine crocodiles are adopting conditional alternative movement tactics, where only the largest males are territory-holders while less competitive males adopt alternative tactics to navigate their social environment. Our findings illustrate how long-term telemetry studies not only can be used to quantify individual behaviour within a natural setting but can also provide insights into the mechanisms and costs of movement tactics among wild animal populations.
References
Argüelles M, Benavides C, Fernández I (2014) A new approach to the identification of regional clusters: hierarchical clustering on principal components. Appl Econ 46:2511–2519. https://doi.org/10.1080/00036846.2014.904491
Baird TA, Baird TD, Shine R (2012) Aggressive transition between alternative male social tactics in a long-lived Australian dragon (Physignathus lesueurii) living at high density. PLoS ONE 7:e41819. https://doi.org/10.1371/journal.pone.0041819
Baker CJ, Franklin CE, Campbell HA, Irwin TR, Dwyer RG (2019) Ontogenetic shifts in the nesting behaviour of female crocodiles. Oecologia 189:891–904. https://doi.org/10.1007/s00442-019-04382-4
Baker CJ, Frère CH, Franklin CE, Campbell HA, Irwin TR, Dwyer RG (2021) Crocodile social environments dictated by male philopatry. Behav Ecol 33:156–166. https://doi.org/10.1093/beheco/arab120
Baker CJ, Frère CH, Franklin CE, Campbell HA, Irwin TR, Dwyer RG (2023) Long-term tracking reveals a dynamic social system. Anim Behav. https://doi.org/10.1016/j.anbehav.2023.02.015
Baldi R, Campagna C, Pedraza S, LeBoeuf BJ (1996) Social effects of space availability on the breeding behaviour of elephant seals in Patagonia. Anim Behav 51:717–724. https://doi.org/10.1006/anbe.1996.0075
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Britton AR, Whitaker R, Whitaker N (2012) Here be a dragon: exceptional size in a saltwater crocodile (Crocodylus porosus) from the Philippines. Herpetol Rev 43:541–546
Campbell HA, Dwyer RG, Irwin TR, Franklin CE (2013) Home range utilisation and long-range movement of estuarine crocodiles during the breeding and nesting season. PLoS ONE 8:e62127. https://doi.org/10.1371/journal.pone.0062127
Campbell HA, Dwyer RG, Wilson H, Irwin TR, Franklin CE (2015) Predicting the probability of large carnivore occurrence: a strategy to promote crocodile and human coexistence. Anim Conserv 18:387–395. https://doi.org/10.1111/acv.12186
Chapman BB, Hulthén K, Blomqvist DR, Hansson L-A, Nilsson J-Å, Brodersen J, Anders Nilsson P, Skov C, Brönmark C (2011) To boldly go: individual differences in boldness influence migratory tendency. Ecol Lett 14:871–876. https://doi.org/10.1111/j.1461-0248.2011.01648.x
Clement P (1987) Movements in rotifers: correlations of ultrastructure and behaviour. In: May L, Wallace R, Herzig A (eds) Rotifer Symposium IV. Springer, Dordrecht, pp 339–359
Clutton-Brock TH (1989) Review lecture: mammalian mating systems. Proc R Soc LondB 236:339–372. https://doi.org/10.1098/rspb.1989.0027
Clutton-Brock TH, Albon SD (1979) The roaring of red deer and the evolution of honest advertisement. Behaviour 69:145–170. https://doi.org/10.1163/156853979x00449
Dalla Rosa L, Secchi ER, Maia YG, Zerbini AN, Heide-Jorgensen MP (2008) Movements of satellite-monitored humpback whales on their feeding ground along the Antarctic Peninsula. Polar Biol 31:771–781. https://doi.org/10.1007/s00300-008-0415-2
Debeffe L, Morellet N, Cargnelutti B, Lourtet B, Bon R, Gaillard JM, Mark Hewison AJ (2012) Condition-dependent natal dispersal in a large herbivore: heavier animals show a greater propensity to disperse and travel further. J AnimEcol 81:1327–1327. https://doi.org/10.1111/j.1365-2656.2012.02014.x
Dwyer RG, Campbell HA, Cramp RL, Burke CL, Micheli-Campbell MA, Pillans RD, Lyon BJ, Franklin CE (2020) Niche partitioning between river shark species is driven by seasonal fluctuations in environmental salinity. FunctEcol 34:2170–2185. https://doi.org/10.1111/1365-2435.13626
Franklin CE, Read MA, Kraft PG, Liebsch N, Irwin SR, Campbell HA (2009) Remote monitoring of crocodilians: implantation, attachment and release methods for transmitters and data-loggers. Mar Freshw Res 60:284–292. https://doi.org/10.1071/MF08153
Fujisaki I, Hart KM, Mazzotti FJ, Cherkiss MS, Sartain AR, Jeffery BM, Beaucahmp JS, Denton M (2014) Home range and movements of American alligators (Alligator mississippiensis) in an estuary habitat. Anim Biotelemetry 2:8. https://doi.org/10.1186/2050-3385-2-8
Grigg G (2001) Crocodilian biology and evolution. Surrey Beatty & Sons, Chipping Norton, NSW
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends EcolEvol 11:92–98. https://doi.org/10.1016/0169-5347(96)81050-0
Haley MP, Deutsch CJ, Le Boeuf BJ (1994) Size, dominance and copulatory success in male northern elephant seals, Mirounga angustirostris. AnimBehav 48:1249–1260. https://doi.org/10.1006/anbe.1994.1361
Hamilton MT, Finger JW, Elsey RM, Mastromonaco GF, Tuberville TD (2018) Corticosterone in American alligator (Alligator mississippiensis) tail scutes: evaluating the feasibility of using unconventional samples for investigating environmental stressors. Gen Comp Endocrinol 268:7–13. https://doi.org/10.1016/j.ygcen.2018.07.008
Hanson JO, Salisbury SW, Campbell HA, Dwyer RG, Jardine TD, Franklin CE (2015) Feeding across the food web: the interaction between diet, movement and body size in estuarine crocodiles (Crocodylus porosus). Austral Ecol 40:275–286. https://doi.org/10.1111/aec.12212
Hertel AG, Niemela PT, Dingemanse NJ, Mueller T (2020) A guide for studying among individual behavioral variation from movement data in the wild. Mov Ecol 8:30. https://doi.org/10.1186/s40462-020-00216-8
Horváth G, Jiménez-Robles O, Martín J, López P, De la Riva I, Herczeg G (2020) Linking behavioural thermoregulation, boldness and individual state in male Carpetan rock lizards. Ecol Evol 10:10230–10241. https://doi.org/10.1002/ece3.6685
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science 348:1221. https://doi.org/10.1126/science.1255642
Kingma SA, Bebbington K, Hammers M, Richardson DS, Komdeur J (2016) Delayed dispersal and the costs and benefits of different routes to independent breeding in a cooperatively breeding bird. Evolution 70:2595–2610. https://doi.org/10.1111/evo.13071
Knapp R, Hews DK, Thompson CW, Ray LE, Moore MC (2003) Environmental and endocrine correlates of tactic switching by nonterritorial male tree lizards (Urosaurus ornatus). HormBehav 43:83–92. https://doi.org/10.1016/S0018-506X(02)00018-1
Lang J (1987) Crocodilian behavior: implications for management. In: Webb GJW, Manolis C, Whitehead PJ (eds) Wildlife Management: Crocodiles and Alligators, Surrey Beatty & Sons / Conservation Commision of the Northern Territory. Chipping Norton, NSW, pp 273–294
Le S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18. https://doi.org/10.18637/jss.v025.i01
Leboeuf BJ (1974) Male-male competition and reproductive success in elephant seals. Am Zool 14:163–176. https://doi.org/10.1093/icb/14.1.163
Lincoln GA (1971) The seasonal reproductive changes in the red deer stag (Cervus elaphus). J Zool 163:105–123. https://doi.org/10.1111/j.1469-7998.1971.tb04527.x
Martin J, van Moorter B, Revilla E, Blanchard P, Dray S, Quenette PY, Allainé D, Swenson JE (2013) Reciprocal modulation of internal and external factors determines individual movements. J AnimEcol 82:290–300. https://doi.org/10.1111/j.1365-2656.2012.02038.x
McElligott AG, Mattiangeli V, Mattiello S, Verga M, Reynolds CA, Hayden TJ (1998) Fighting tactics of fallow bucks (Damadama, Cervidae): reducing the risks of serious conflict. Ethology 104:789–803. https://doi.org/10.1111/j.1439-0310.1998.tb00112.x
Melzheimer J, Strew S, Wasiolka B, Fischer M, Thalwitzer S, Heinrich SK, Weigold A, Hofer H, Wachter B (2018) Queuing, takeovers, and becoming a fat cat: long-term data reveal two distinct male spatial tactics at different life-history stages in Namibian cheetahs. Ecosphere 9:e02308. https://doi.org/10.1002/ecs2.2308
Meyer L, Chambers S, Gervais C, Pethybridge H, Beckmann C, Bruce B, Huveneers C (2021) The use of muscle lipids and fatty acids to assess shark diet and condition. J Fish Biol 98:566–571. https://doi.org/10.1111/jfb.14602
Michelangeli M, Payne E, Spiegel O, Sinn DL, Leu ST, Gardner MG, Sih A (2022) Personality, spatiotemporal ecological variation and resident/explorer movement syndromes in the sleepy lizard. J AnimEcol 91:210–223. https://doi.org/10.1111/1365-2656.13616
Modig AO (1996) Effects of body size and harem size on male reproductive behaviour in the southern elephant seal. AnimBehav 51:1295–1306. https://doi.org/10.1006/anbe.1996.0134
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE (2008) A movement ecology paradigm for unifying organismal movement research. P Natl Acad Sci USA 105:19052–19059. https://doi.org/10.1073/pnas.0800375105
Newton I (2008) The migration ecology of birds. Academic Press, London
Nilsen FM, Dorsey JE, Lowers RH, Guillette LJ, Long SE, Bowden JA, Schock TB (2017) Evaluating mercury concentrations and body condition in American alligators (Alligator mississipiensis) at Merritt Island National Wildlife Refuge (MINWR), Florida. Sci Total Environ 607-608:1056–1064. https://doi.org/10.1016/j.scitotenv.2017.07.073
Ridley AR, Raihani NJ, Nelson-Flower MJ (2008) The cost of being alone: the fate of floaters in a population of cooperatively breeding pied babblers Turdoides bicolor. J Avian Biol 39:389–392. https://doi.org/10.1111/j.0908-8857.2008.04479.x
Rimbach R, Blanc S, Zahariev A, Pillay N, Schradin C (2019) Daily energy expenditure of males following alternative reproductive tactics: solitary roamers spend more energy than group-living males. Physiol Behav 199:359–365. https://doi.org/10.1016/j.physbeh.2018.12.003
Rosenblatt AE, Heithaus MR (2011) Does variation in movement tactics and trophic interactions among American alligators create habitat linkage? J Anim Ecol 80:786–798. https://doi.org/10.1111/j.1365-2656.2011.01830.x
Rosenblatt AE, Heithaus MR, Mazzotti FJ, Cherkiss M, Jeffery BM (2013) Intra-population variation in activity ranges, diel patterns, movement rates and habitat use of American alligators in a subtropical estuary. Estuar Coast Shelf Sci 135:182–190. https://doi.org/10.1016/j.ecss.2013.10.008
Schradin C, Scantlebury M, Pillay N, König B (2009) Testosterone levels in dominant sociable males are lower than in solitary roamers: physiological differences between three male reproductive tactics in a sociably flexible mammal. Am Nat 173:376–388. https://doi.org/10.1086/596535
Seebacher F, Franklin C, Read M (2005) Diving behaviour of a reptile (Crocodylus johnstoni) in the wild: interactions with heart rate and body temperature. Physiol Biochem Zool 78:1–8. https://doi.org/10.1086/425192
Smetzer JR, Paxton KL, Paxton EH (2021) Individual and seasonal variation in the movement behavior of two tropical nectarivorous birds. Mov Ecol 9:36. https://doi.org/10.1186/s40462-021-00275-5
Spiegel O, Leu ST, Sih A, Godfrey SS, Bull CM (2015) When the going gets tough: behavioural type-dependent space use in the sleepy lizard changes as the season dries. Proc R Soc B 282:20151768. https://doi.org/10.1098/rspb.2015.1768
Strickland BA, Gastrich K, Beauchamp JS, Mazzotti FJ, Heithaus MR (2022) Effects of hydrology on the movements of a large-bodied predator in a managed freshwater marsh. Hydrobiologia 849:861–878. https://doi.org/10.1007/s10750-021-04764-x
Strickland BA, Vilella FJ, Belant JL (2016) Scale-dependent habitat selection and size-based dominance in adult male American alligators. PLoS ONE 11:e0161814. https://doi.org/10.1371/journal.pone.0161814
Stuber EF, Carlson BS, Jesmer BR (2022) Spatial personalities: a meta-analysis of consistent individual differences in spatial behavior. Behav Ecol 33:477–486. https://doi.org/10.1093/beheco/arab147
Taborsky M, Oliveira RF, Brockmann HJ (2008) The evolution of alternative reproductive tactics: concepts and questions. In: Alternative Reproductive Tactics: an integrative approach. Cambridge University Press, Cambridge, pp 1–21
Webb G, Smith A (1987) Life history parameters, population dynamics and the management of crocodilians. In: Webb GJW, Manolis C, Whitehead PJ (eds) Wildlife management: Crocodiles and alligators. S. Beatty & Sons, Chipping Norton, NSW, pp 199–210
Webb GJW, Messel H (1978) Morphometric Analysis of Crocodylus porosus from the north coast of Arnhem Land, northern Australia. Aust J Zool 26:1–27. https://doi.org/10.1071/ZO9780001
Wikelski M, Carbone C, Trillmich F (1996) Lekking in marine iguanas: female grouping and male reproductive strategies. Anim Behav 52:581–596. https://doi.org/10.1006/anbe.1996.0199
Wolf JBW, Trillmich F (2007) Beyond habitat requirements: individual fine-scale site fidelity in a colony of the Galapagos sea lion (Zalophus wollebaeki) creates conditions for social structuring. Oecologia 152:553–567. https://doi.org/10.1007/s00442-007-0665-7
Acknowledgements
We acknowledge the Taepadhigi, Tjumgundji, and Warranggu peoples as the traditional owners on whose land we conducted our research. We thank all members of the Australia Zoo croc team for their assistance in the capture and release of estuarine crocodiles, and Australia Zoo, Rio Tinto, and the Mapoon Land and Sea Rangers for servicing acoustic receivers. We thank the reviewers for their time and for the helpful comments they provided, which allowed us to improve the quality of the manuscript.
Data accessibility
Data and code are available on UQ eSpace (https://doi.org/10.14264/9b029a6), and data is available on the Acoustic Animal Tracking Database (https://animaltracking.aodn.org.au) of the Integrated Marine Observing System (IMOS, www.imos.org.au), a national collaborative research infrastructure supported by the Australian government.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by the Australian Research Council linkage scheme with Australia Zoo and CSIRO as industry partners (LP140100222). Donations toward research and field costs were also received from the Australia Zoo Wildlife Warriors (https://wildlifewarriors.org.au/conservation-projects/crocodile-research).
Author information
Authors and Affiliations
Contributions
KEB, CJB, RGD, HAC, and CEF conceived the ideas and designed the methodology. All authors collected the data. KEB, CJB, and RGD led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Ethics approval
All procedures were carried out with approval from The University of Queensland Animal Ethics Committee (SIB/302/08/ ARC, SBS/204/11/ARC/AUST ZOO (NF), SBS/215/14/AUST ZOO/ARC) and Queensland Environment Protection Agency Permits (WISP00993703, WISP05268508, WISP13189313). All applicable national and institutional guidelines for the use of animals were followed.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by T. Madsen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barham, K.E., Baker, C.J., Franklin, C.E. et al. Conditional alternative movement tactics in male crocodiles. Behav Ecol Sociobiol 77, 31 (2023). https://doi.org/10.1007/s00265-023-03303-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03303-z