Abstract
The structural condition of feathers may generally have a decisive role in shaping the color properties of the plumage. However, the information content of structurally mediated color differences is poorly known. This makes it particularly hard to determine the meaning of color variation in pigment-free white plumage patches. The white wing patch of the collared flycatcher (Ficedula albicollis) is an important sexual trait, and changes in its reflectance are partly due to macrostructural condition. We used 2 years of macrostructural, reflectance, and breeding data from both sexes to examine whether wing patch macrostructure lends information content to actual reflectance in terms of reproductive effort and success. Macrostructure strongly predicted actual reflectance in males but only weakly in females. Furthermore, in males, feather vane width was related positively to current year reproductive effort, and negatively to previous year reproductive effort. This indicates that macrostructurally mediated reflectance attributes may inform the receiver not only of actual reproductive capacity but also of individual quality via reproductive costs.
Significance statement
Coloration of animals takes a central place in their communication and in advertising reproductive abilities. Although white plumage is widespread among animals, usually we have little knowledge on how its structure is linked to reproduction. We investigated this link in a wild population of collared flycatchers. We demonstrated that white feather structure was related to coloration and with current year and previous year reproductive capabilities in males. Our results suggest that white feather structure has the potential to connect reproductive costs with coloration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expression of plumage color traits can be the target of sexual selection and convey information about individual quality (Hill 2006). Although the presence of white patches is immensely widespread among birds, in contrast to the more detailed knowledge on colored plumage, we still have scant information on how white plumage conveys information about the bearer’s quality or condition.
Animal colors result from two different basic mechanisms. Structurally based colors arise from microstructures scattering the incident light, while pigment-based colors are the result of light absorption, but these two mechanisms oftentimes produce colors jointly together (Shawkey and D'Alba 2017). Considering structural chromatic colors (i.e., the reflectance curves have at least one peak and/or plateau), it is suggested that the presence of pigments is necessary for creating the observed iridescent (angle-dependent) or non-iridescent color (Shawkey and D'Alba 2017). Additionally, in these cases, the microstructures are highly organized, resulting in coherent scattering of light and constructive interference of certain wavelengths (e.g., Prum and Torres 2003).
With a few exceptions (e.g., Insausti and Casas 2008), reflectance of achromatic white integuments is purely structurally based, which means that the reflectance characteristics are determined without the contribution of pigment molecules. In most of these cases, we observe incoherent (i.e., not wavelength-selective) light scattering originating from disordered tissue structures (Shawkey and Hill 2005; Wiersma 2013). In birds, recent findings have started to open a broader view on how the feather structures at both the micro- and macroscale levels modify the reflectance curves of white feathers. Macrostructure is mostly the mesh topology of hierarchically branched feather units (barbs and barbules) that tailors the visible feather shape, while microstructure is characterized by the inner and surface features of the above units. At the among-species level (including 61 species), Igic et al. (2018) showed that substantial differences in reflectance of white body contour feathers were positively related to the density and thickness of barbs and barbules, barbule length, and barb roundedness. Stuart-Fox et al. (2018) comparatively investigated 50 bird species and found that higher brightness in white body contour feathers between species was associated with more densely packed barbules and rounder barbs. Within species, at the individual level, it was found that short-term temporal changes in brightness and relative UV reflectance of white feathers could be related to changes in barb angle and vane width respectively (Laczi et al. 2021). These results highlight that macrostructure could play an important role in shaping white feather reflectance.
The above studies might be the first steps towards digging out the hidden direct links between the reflectance of white ornaments and their information content. In parallel with this, the information content of white plumage has been tested only in a few cases yet. According to the available studies, spectral properties of white feathers can be associated with hormone levels (Cantarero et al. 2017) in the pied flycatcher (Ficedula hypoleuca), eggshell pigmentation (Badás et al. 2017) in the blue tit (Cyanistes caeruleus), immune state and body mass change (Hanssen et al. 2006) in the common eider (Somateria mollissima), hematocrit level (Bettega et al. 2013) in the Eurasian eagle-owl (Bubo bubo), cell-mediated immune response (Zanollo et al. 2012) in the diamond firetail (Stagonopleura guttata), stress resilience and social interaction frequency (Taff et al. 2019) in the tree swallow (Tachycineta bicolor), and mate preference, social dominance (Woodcock et al. 2005), and reproductive success (Doucet et al. 2005) in the black-capped chickadee (Poecile atricapillus). Furthermore, it has been experimentally demonstrated in the dark-eyed junco (Junco hyemalis) that white feather brightness can be negatively affected by nutritional stress before/during molt and molting speed (McGlothlin et al. 2007).
Feather quality, which is generally one of the principal determinants of the actual plumage coloration, can be negatively affected by hurried and/or overlapping molt (e.g., Dawson et al. 2000; Echeverry-Galvis and Hau 2013; Møller and Nielsen 2018). It is known that there could be trade-offs between the costly reproductive effort before molt and future color ornament expression (developed during molt) in the next breeding attempt long after molt, for example in the case of the melanin-based black badge size in the house sparrow (Passer domesticus) and in the structural-based UV-blue brightness in the eastern bluebird (Sialia sialis) (Griffith 2000; Siefferman and Hill 2005, respectively). Considering the reflectance determining mechanisms described above, these trade-offs could be mediated by feather macrostructural quality. However, the potential effects of reproduction on the macrostructural component of feather coloration have not been investigated previously. Furthermore, the ornamentation of an individual may affect the parental investment of its social mate as it has been demonstrated, for example, in blue tits, where males showed reduced feeding trip numbers and prey size when mated with experimentally UV-reduced (i.e., less attractive) females (Mahr et al. 2012), or in collared flycatchers (Ficedula albicollis), where male feeding rate was negatively related to the structurally based plumage brightness of the partner (Laczi et al. 2017). Structurally based reflectance may signal past reproduction or physical state (reproductive capacity) via either the quality of the feathers grown at molt or their abrasion since molt. Therefore, the state of the macrostructure of the feather which affects reflectance may also correlate with measures of the current reproductive bout due either to their own or their partner’s reproductive investment. In other words, feather macrostructural parameters may also link plumage reflectance to current reproductive success.
In this study, we aimed to explore the relationship between past and current reproductive effort in connection with current feather macrostructure and reflectance in a wild population of collared flycatchers. This species is sexually dichromatic, and exhibits a melanin pigmented black (male) and brown (female) plumage with pigment-free white patches (forehead patch mostly in males, wing patch in both sexes). Previous research in the studied population has indicated that sexual selection might play a role in driving the expression of across-plumage reflectance properties in both sexes as these were related to mating patterns, condition, and past and current reproductive effort (Laczi et al. 2011, 2013, 2017). Additionally, not only reflectance properties, but the extent of white unpigmented plumage patches could also reflect previous or current reproductive parameters. For example, patch size could be negatively related to past reproductive investment, and positively to current reproductive investment (e.g., Gustafsson et al. 1995; Hegyi et al. 2008; Kötél et al. 2016). Additionally, in females, a strong association was recently detected between the short-term (within-season) changes of reflectance properties (brightness, UV chroma) and feather macrostructure in the white wing patch (Laczi et al. 2021).
In the present study, we expected a decreased investment in feather production of the white wing patch after higher reproductive investment (clutch size, fledging number) in the previous year. In parallel with this, we also predicted negative relationships between current reproductive investment and feather quality due to the presumed trade-off between reproductive activities and self-maintenance (Cucco and Malacarne 1997), taking into account the temporal and energetic costs of maintenance (e.g., Walther and Clayton 2005; Viblanc et al. 2011).
Methods
Data collection
The collared flycatcher is a long-distance migratory, cavity-nesting passerine species, molting twice a year, in summer and in winter. Only the summer molt between breeding and migration includes the replacement of the primary feathers. Hence, these feathers are not re-grown during the partial winter molt in Africa (Demongin 2016), which means that they can be measured during the reproductive season in the following year.
We collected breeding data from 2019 to 2021 and also took reflectance and photographic records of breeding birds in 2020 and 2021 in our artificial nest-box plots in the Pilis-Visegrádi Mountains, Duna–Ipoly National Park, Hungary (47°43′N, 19°01′E; Török and Tóth 1999), during the nestling-feeding period of this species (late May-early June). We checked nest boxes regularly (every 5 days) in order to determine the date of clutch initiation (i.e., laying date of the first egg relative to the yearly median laying date). We distinguished three measures of reproductive investment: early, late, and total reproductive investment. We characterized the early reproductive investment with clutch size (i.e., the number of eggs laid), for multiple reasons. First, there is minimal hatching failure and early nestling mortality in our population, so the number of eggs strongly correlates with the number of nestlings reared in the early nestling stage (clutch size-hatching number for the current year data set of females: Spearman correlation rs = 0.80, p < 0.001, N = 270; males: rs = 0.80, p < 0.001, N = 227; note that slightly fewer males than females were caught due to logistic reasons). Hence, clutch size gives an adequate approximation of hatched nestling number even in case we do not know the exact number (current year data set, females: N = 30, males: N = 22; previous year data set, females: N = 4, males: N = 7). Second, in altricial bird species, typically, males do most of the feeding work in the early nestling stage because females are still exclusively brooding the ectothermic young (e.g., Perrins 1979; Jenkins et al. 2021). Brooding continues up to 6 days of nestling age in the collared flycatcher. Additionally, it is likely that a greater number of offspring does not increase the energy loss of the brooding female as much as that of the male that performs most of the feeding in this period when chicks are ectothermic and cannot regulate their own temperature. Therefore, in our context, clutch size may specifically indicate the reproductive investment of males in the early nestling stage. The number of fledglings was used as a measure of total reproductive investment, i.e., the number of young raised from hatching to near independence. Unsuccessful nests were omitted from the coding of this variable because total nest failure was in most cases due to reasons other than reproductive investment (typically predation), and such failure also occurred relatively late when males had already made much of their parental investment. Finally, the number of fledglings corrected for clutch size was used to quantify late reproductive investment. This mainly indicates the additional pressure placed on the parents in the second half of the nestling period (in accordance with this, more than two-third of the events of non-predation-related reduction in nestling number detected in the relevant years occurred in the late nestling stage; our unpublished data). After omitting the unsuccessful nests, fledgling number was corrected for clutch size using the year-corrected values of both variables.
Birds were captured in their nest box at 8–10-day-old offspring age and marked with individually numbered rings (Aranea, Poland, standard rings of the Hungarian Bird Ringing Centre). For ethical reasons and to prevent unnecessary disturbance to the birds and to avoid potential effects caused by wet plumage (e.g., Shawkey et al. 2011), we did not capture or handle birds in rainy weather. We measured reflectance of the wing patch using a USB2000 spectrometer, DH-2000 light source, R400-7 bifurcated micron fiber-optic probe detector (perpendicular to the measured surface, at a distance of 3 mm, with a black sheath to exclude ambient light), WS-1-SS white standard, and the OOIBase32 software (all from Ocean Optics Europe). From these spectra (see Fig. 1), we later calculated brightness (average intensity between 320 and 700 nm, R320–700) and ultraviolet (UV) chroma (relative UV intensity, R320–400/R320–700). For more details of wing patch spectral measurements, see Laczi et al. (2021).
Mean reflectance spectra of the white wing patch of the collared flycatcher. Data were collected in 2020–2021. Solid line, females; dashed line, males. Shaded areas represent SD. The figure was made using the Pavo R package (Maia et al. 2019)
After spectrometric measurements, we took photographs of the white patch of the sixth primary, using a Nikon D5600 camera with a Sigma 105 mm 1:2.8 DG Macro HSM lens with a fixed distance between the front lens and wing surface. Later, using RawTherapee v5.6 (http://www.rawtherapee.com/), we converted RAW files without any image correction into jpg which is an analyzable file format. We used the Scanning Probe Image Processor (Image Metrology Inc.) software to quantify macrostructural variables (Fig. 2). We measured average barb angle to the nearest 0.01 degree as determined relative to the rachis, alongside the proximal, uncurved, longer section of the barb, averaged from measures of five consecutive barbs. Average outer vane width was used to estimate the reflective surface size. This variable was calculated as the perpendicular distance between the rachis and the vane edge (to the nearest 0.01 mm), averaged from three separate measurements within the patch. Since the width and angle measurements in the same measurement points showed very high repeatability in a previous study on this population (Laczi et al. 2021) and taking into account that the same person (ML) measured the samples to avoid the interobserver error in the previous and the present study, only one set of measures were taken for each sample in this study. As vane width and barb angle correlated very strongly in the present data set (females: r = 0.68, p < 0.001, N = 301; males: r = 0.72, p < 0.001, N = 250), we used only vane width for the analyses as it reflects multiple different aspects of the vane structure, including angle (see, e.g., Feo et al. 2016). We collected the spectrometric and patch morphological data from the same wing in each bird.
A photograph of the 6th primary of a female collared flycatcher for illustrating the measurements of wing patch macrostructure. The narrower, outer vane with the visible white wing patch is at the bottom. Letters indicate the measurements of (a) vane width, (b) barb angle to the rachis, and (c) barb length. For more details, see the text
We quantified the visible feather wear (damage) on the outer vane on the white part of the most worn primary of the wing patch (in most cases, this was the fourth primary) as a binary variable. A feather was assigned as undamaged if the white outer vane width was not narrower than the half-width of the melanized feather segment immediately above the white patch, while otherwise it was assigned as damaged. To minimize observer bias, reflectance and macrostructure measures were taken without knowing the exact identity (ring number) of the birds; however, it was not possible to record spectral data blind with regard to sex because our study involved focal animals in the field. All measurements were performed without knowledge of the reproductive performance of individuals.
Statistical analysis
From the analyses, we excluded yearling males because they were represented in low numbers in our data set, and in parallel with this, their wing patch size and reflectance markedly differs from older males (Laczi et al. 2021). Besides, we excluded birds engaged in a socially polygynous relationship (i.e., males that were trapped at two nest boxes with overlapping nestling-rearing phases, and their mates, see Herényi et al. 2014), with re-nesting events, or if involved in experiments. In the case of birds measured in both years, we used their spectral and macrostructural measures only from the second year, in order to avoid pseudoreplication. For the analyses of fledging numbers, we used only those individuals whose nest was not subject to predation or nest abandonment.
We analyzed females and males separately because of their qualitatively different reflectance (Laczi et al. 2011). We used Pearson’s correlations for checking collinearity between vane width and barb angle (see above), and brightness and UV chroma. For the comparisons of sexes, we used Student’s t tests in the cases of macrostructure and reflectance, whereas feather wear was analyzed by binary logit regression model using feather wear as the dependent variable, and sex as categorical predictor. Before the following analyses, vane width, barb angle, current and previous clutch size, and fledging success in both sexes were standardized to a mean of zero and a standard deviation of one within each year. As proxies of the previous year and the current year late reproductive investment, we used the regression residuals of fledging number on clutch size. Association of macrostructure with feather wear was analyzed using Student’s t tests, and its associations with reflectance and reproductive investment were checked by Pearson’s correlations. Laying date was log-transformed in order to achieve normality. Analyses were performed in Statistica v5.5 (StatSoft Inc., Dell, Rock Round, TX, USA). We applied false discovery rate (FDR) correction for controlling the number of tests performed under the same hypothesis (Benjamini and Hochberg 1995; Pike 2011).
Results
Brightness and UV chroma were not correlated with each other (females: r = − 0.09, p = 0.13, N = 299; males: r = 0.05, p = 0.47, N = 239).
Females expressed narrower vane width (t = 3.85, df = 549, p = 0.0001, effect size r = 0.15; mean ± SD females: 1.15 ± 0.21; males: 1.22 ± 0.21), and less pronounced brightness than males (t = 19.10, df = 536, p < 0.0001, effect size r = 0.63; females: 31.66 ± 4.41; males: 39.27 ± 4.81) and UV croma (t = 2.64, df = 536, p = 0.008, effect size r = 0.11; females: 0.83 ± 0.06; males: 0.84 ± 0.05). Visible feather wear did not differ between sexes (Wald χ2 = 0.003, df = 508, p = 0.95, effect size r = 0.002; number of undamaged/damaged individuals in females: 196/77; in males: 170/66).
Vane width was negatively associated with binary feather wear in both females (t = 5.35, df = 1271, p < 0.0001, effect size r = 0.56; mean ± SD undamaged: 0.20 ± 0.95, damaged: − 0.50 ± 1.00) and males (t = 3.38, df = 1234, p = 0.0008, effect size r = 0.46; undamaged: 0.14 ± 1.00, damaged: − 0.35 ± 0.97) (Fig. 3). However, in females, vane width correlated weakly with brightness (r = 0.12, p = 0.042, N = 299) and there was no correlation with UV chroma (r = 0.05, p = 0.37, N = 299), whereas in males reflectance variables showed stronger positive connections with vane width (brightness: r = 0.24, p < 0.001, N = 239; UV chroma: r = 0.39, p < 0.001, N = 239) (Fig. 4). As macrostructure in females predicted reflectance properties only slightly at the individual level, here we did not present the correlations of macrostructure with reproductive effort in females (see for Supplementary Information on these results).
In males, vane width positively correlated with current fledging number (r = 0.18, p = 0.010, N = 209) (Fig. 5) and corrected fledging number (r = 0.15, p = 0.021, N = 209). The other relationships were not significant (laying date: r = − 0.12, p = 0.051, N = 249, this is far from significant after FDR-correction (adjusted significance threshold q = 0.025); clutch size: r = 0.06, p = 0.33, N = 249).
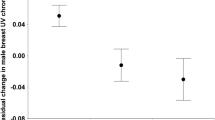
Considering past reproductive investment, in males, we found a negative correlation between current year vane width and previous year clutch size (r = − 0.25, p = 0.007, N = 117) (Fig. 6) but no other significant relationships emerged (laying date: r = 0.11, p = 0.22, N = 117; fledging number: r = − 0.15, p = 0.13, N = 106; corrected fledging number: r = − 0.02, p = 0.82, N = 106). After FDR correction (q = 0.0125), the significant relationships indicated above remained significant.
Discussion
In this study on collared flycatchers, we explored associations of white wing patch macrostructure with feather wear, reflectance, and sex, and in males with current and past reproductive effort.
When considering feather wear, it is important to highlight its strong association with vane width, which means that the measured white vane width is not exclusively an inherent attribute of the intact feather. In other words, a narrower white vane is partly the consequence of feather wear which probably causes vane narrowing because damages in the interlocking structures increase overlaps between barbs (see later). Another mechanism could be the breakage of barb tips, like in primaries of the barn owl (Tyto alba) (Piedrahita et al. 2021), but in collared flycatchers this happens very rarely and involves only a few non-adjacent, single barbs on the investigated segment of the primary vane (our unpublished data).
Vane width was greater in males, which is in concordance with the more pronounced coloration in this sex, as the vane functions as a reflective surface area (Laczi et al. 2021). The greater vane width of males can be caused by actually larger barb length through more keratin intake and/or by the barbs starting to curve more distally as the curved paths could have an influence on vane width (Feo et al. 2016). Between sexes, feather wear probably could not be the principal reason for the described difference as visible wear did not differ between the two groups. In parallel with this, between individuals within sexes, barb angle and vane width showed a positive correlation. Thus, similar to the long-billed curlew (Numenius americanus), a species with the same barb curvature type as in our species, the vane width could be partially and positively determined by the angle of the straight barb section, but there may also be a contribution by the angle of the curved section we did not measure (Feo et al. 2016).
Within sexes, the brightness and the UV chroma of the white patch were strongly positively correlated with vane width in males, but in females, we found a weak connection and only for brightness. This means that a wider wing patch was associated with higher brightness in both sexes and higher UV chroma in males, possibly because a larger surface area could increase the overlapping white feather layers between adjacent primaries. However, as the vane width was narrower in females, this phenomenon may be more modest, leading to weaker relationships between the macrostructure and reflectance. Additionally, in a previous study (Laczi et al. 2021) on collared flycatcher females, there were correlations between short-term reflectance changes and macrostructural changes only in the early breeding period (from incubation to 2-day-old nestling age), and these were absent in a later stage, when we collected data for the present study (8–10-day-old nestling age). Hence, there could be stage-specific abrasion effects that cause the weakening of the dependence of color expression on the measured macrostructural components in females. In addition, the female primary may be more sensitive to certain damages, for example, suffering from more pronounced barbule loss through time (although this is more pronounced and visible at the distal part of the primaries, our personal observations), which could have stronger effects on reflectance variance in later periods than the large-scale structural components we measured here.
In males, considering the apparent effects of previous year reproductive investment, there was a negative relationship between current vane width and previous year early investment. The effect was in the same direction but non-significant for total investment, and even weaker for late investment. The detected correlation may be the consequence of a trade-off between the early investment and the investigated phenotypic character. This picture is highly logical as males do most of the feeding work in the early nestling stage, when clutch size adequately depicts investment, but they contribute a much smaller proportion of work compared to the female in the late stage, when fledgling number has additional explanatory value due to late nestling mortality.
Accordingly, wing patch feather condition is apparently sensitive to the costs of past reproduction, and this sensitivity may contribute to the information content of wing patch reflectance. We presumed that this pattern could be the result of a process whereby males that had raised a larger clutch might not have been able to develop primaries with wider outer vanes. During feather replacement, birds face substantial challenges including costs of energy (e.g., Murphy 1996), loss of time (Jenni and Winkler 2004), increased predation vulnerability (Slagsvold and Dale 1996), and reduced flight performance (Swaddle and Witter 1997). Hence, in many species, molt is more or less separated temporally from reproduction to reduce trade-offs between these life-history events (Barta et al. 2008). However, even in cases with no or moderate overlap with molt, reproductive performance may significantly influence the quality of feathers grown in the post-breeding molt. For example, delayed breeding schedule or larger clutch size could cause deferred molt (Siikamäki et al. 1994; Hemborg et al. 2001), from which a major time constraint on molting duration could emerge, especially in light of the proximity of autumn migration. Individuals with overlapping breeding and molt may grow wing feathers with lower mass and shorter length (Echeverry-Galvis and Hau 2013); furthermore, lower food quality may also result in the same patterns (Pap et al. 2008). Additionally, a consequence of accelerated feather replacement is the production of wing feathers more prone to feather wear (Dawson et al. 2000; Møller and Nielsen 2018; Serra 2001). Based on these findings, we suggest that male collared flycatchers that raised larger clutches in the previous year probably faced pronounced energy constraints because of their poorer physical state. The poor condition could lead to growing feathers with lower keratin deposition, resulting in actually narrower vane due to the shorter barbs developed. In addition, the abrasion proneness of the developed feathers may also have been affected by the condition at molt, thereby changing the feather structure by the next breeding season. This means that breeding performance could have a long-lasting effect on vane width or its abrasion so that vane width has the potential to signal an individual’s past condition in the long term, which could in turn become important in reflectance information content during the courtship period.
Vane width of males was not significantly related to early reproductive investment in the current year but significantly positively related to total and late reproductive investment. This suggests that less worn males (and their mates) were more successful in nestling rearing to independence. Therefore, male physiological condition and associated reproductive capacity in the late nestling stage (possibly also including female differential investment) may have influenced both final reproductive success and feather wear state of the wing patch. That is, it is probably not reproductive investment that influenced current year feather wear state but rather the two might have had a common external driver. Therefore, wing patch abrasion state in the late nestling stage (and indirectly wing patch brightness) may indicate physiological state and nestling rearing capacity, and it may therefore be used by females to adjust their reproductive investment to male state. Experimental studies of the contribution of male and female parental care to this pattern are needed to draw firmer conclusions.
It is unavoidable to take into account some aspects of mechanical properties of flight feathers in order to draw a comprehensive picture. Studies indicated that the lowest level of the macrostructural hierarchy, the barbules, takes a substantial share of maintaining simultaneously the robust stability and dynamic pliability of the intact flight feathers (Ennos et al. 1995; Chen et al. 2020). Interlocking bow barbules and hook barbules provide the adhesion of adjacent barbs, and the intact organization of this adhesion is essential for preventing irreversible damages to feathers (see Kovalev et al. 2014). Additionally, a certain barbule spacing is also necessary for appropriate functioning (Sullivan et al. 2017). Due to the flight feather design, barbules not only interlock the adjacent barbs, but also keep those at a certain distance from each other. Hence, missing or damaged barbules, or injuries to the interlocking system, potentially lead to undesirable overlaps between the barbule surfaces carried by individual barbs, which reduces the functionality of the flight feather. In many cases, deformations in barb arrangement are reduced due to self-repairing mechanisms of the vane structure (Kovalev et al. 2014; Zhang et al. 2018). However, even if the damages are reversible, only a good-quality feather has the potential for self-healing (re-zipping the overlapped or separated barbs), and active intervention by preening behavior or plumage shaking is probably also essential for this (Kovalev et al. 2014). It is highly possible that barbules in the periphery of the feather vane in collared flycatcher males are more vulnerable to external damage, especially when they are not in interlocked position. These damages could cause more extensive overlaps (along longer barb segments) between barbule surfaces at the distal part of the barbs (our field observations), which reduces the apparent width of the vane, leading to reduced expression in reflectance properties. Thus, the narrower vane will be associated with less exaggerated brightness and UV chroma (for detailed explanation on the relationship of reflectance with vane width, see Laczi et al. 2021), which allows conspecifics to visually evaluate the structural condition of the plumage.
The revealed pattern, namely that total and late reproductive investment of the current breeding attempt were positively related to vane width, is in accordance with the above details. This is especially true if we also consider an additional phenomenon, namely that the behavior which contributes to feather structure maintenance (such as grooming, pecking, preening) is a highly time- and energy-consuming activity (see Clayton and Cotgreave 1994; Walther and Clayton 2005). Therefore, it requires time and energy freed from other activities (e.g., Redpath 1988; Burger 1997; Cucco and Malacarne 1997). Hence, only males in good physical condition could invest much energy in actively maintaining a structurally intact feather while also rearing more offspring, which presumably means a greater energetic cost, since in our population, nestling feeding rate of parents is higher in enlarged broods (Laczi et al. 2017). Additionally, a male with the capability of foraging more effectively can save time and energy (Norberg 1977), which can be allocated to more frequent feather maintenance. According to this, vane width could reflect some aspects of the actual condition of an individual. Certainly, we cannot exclude alternative, mutually not exclusive explanations. It might be possible that in larger broods, one sex of the parents takes the direct parental duties with relatively more enthusiasm than its social pair, as demonstrated, for example, in male mountain chickadees (Poecile gambeli) with regard to nestling feeding visits (Grundel 1987). In our case when the male of a larger brood partly refrains from these duties, leaving them to the female, this male could spend more time on other activities such as preening, resulting in more intact feathers. However, this scenario is probably not applicable in this population as we formerly found no such differences in the sharing of parental behavior among broods, independently of brood size (Laczi et al. 2017; ML et al. unpublished data).
Our study indicates a possible long-term effect of the past reproductive event on current feather condition and therefore reflectance. In addition, due to the costs of keeping the feathers in good shape, the degree of feather intactness, and the color expression affected by this, could honestly indicate the bearer’s current condition. Finally, these relationships altogether may suggest a potential causal connection between feather reflectance attributes and reproductive effort through the contribution of feather macrostructure.
Data availability
The datasets generated and/or analyzed during the current study is available in the Dryad Digital Repository, https://doi.org/10.5061/dryad.kprr4xh6b (Laczi et al. 2022).
References
Badás EP, Martínez J, Rivero-de Aguilar J, Stevens M, van der Velde M, Komdeur J, Merino S (2017) Eggshell pigmentation in the blue tit: male quality matters. Behav Ecol Sociobiol 71:57. https://doi.org/10.1007/s00265-017-2286-4
Barta Z, McNamara JM, Houston AI, Weber TP, Hedenström A, Fero O (2008) Optimal moult strategies in migratory birds. Phil Trans R Soc B 363:211–229. https://doi.org/10.1098/rstb.2007.2136
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57:289–300
Bettega C, Campioni L, Del Mar DM, Lourenco R, Penteriani V (2013) Brightness features of visual signaling traits in young and adult Eurasian eagle-owls. J Raptores 47:197–207. https://doi.org/10.3356/JRR-12-00002.1
Burger J (1997) Effects of oiling on feeding behavior of sanderlings and semipalmated plovers in New Jersey. Condor 99:290–298
Cantarero A, Laaksonen T, Järvistö PE, López-Arrabé J, Gil D, Moreno J (2017) Testosterone levels in relation to size and UV reflectance of achromatic plumage traits of female pied flycatchers. J Avian Biol 48:243–254. https://doi.org/10.1111/jav.01032
Chen Q, Pugno NM, Li Z (2020) The rotation toughening mechanism of barb–barbule joint in the barb delamination of feathers. Acta Mech 231:1173–1186. https://doi.org/10.1007/s00707-019-02566-w
Clayton DH, Cotgreave P (1994) Comparative analysis of time spent grooming by birds in relation to parasite load. Behaviour 131:171–187
Cucco M, Malacarne G (1997) The effect of supplemental food on time budget and body condition in the black redstart Phoenicurus ochruros. Ardea 85:211–221
Dawson A, Hinsley SA, Ferns PN, Bonser RC, Eccleston L (2000) Rate of moult affects feather quality: a mechanism linking current reproductive effort to future survival. Proc R Soc Lond B 267:2093–2098. https://doi.org/10.1098/rspb.2000.1254
Demongin L (2016) Identification guide to birds in the hand. Privately published, Beauregard-Vendon, pp 316–317
Doucet SM, Mennill DJ, Montgomerie R, Boag PT, Ratcliffe LM (2005) Achromatic plumage reflectance predicts reproductive success in male black-capped chickadees. Behav Ecol 16:218–222. https://doi.org/10.1093/beheco/arh154
Echeverry-Galvis MA, Hau M (2013) Flight performance and feather quality: paying the price of overlapping moult and breeding in a tropical highland bird. PLoS One 8:e61106. https://doi.org/10.1371/journal.pone.0061106
Ennos A, Hickson J, Roberts A (1995) Functional morphology of the vanes of the flight feathers of the pigeon Columba livia. J Exp Biol 198:1219–1228
Feo TJ, Simon E, Prum RO (2016) Theory of the development of curved barbs and their effects on feather morphology. J Morphol 277:995–1013. https://doi.org/10.1002/jmor.20552
Griffith SC (2000) A trade-off between reproduction and a condition-dependent sexually selected ornament in the house sparrow Passer domesticus. Proc R Soc Lond B 267:1115–1119
Grundel R (1987) Determinants of nestling feeding rates and parental investment in the Mountain Chickadee. Condor 89:319–328
Gustafsson L, Qvarnström A, Sheldon BC (1995) Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375:311–313
Hanssen SA, Folstad I, Erikstad KE (2006) White plumage reflects individual quality in female eiders. Anim Behav 71:337–343. https://doi.org/10.1016/j.anbehav.2005.04.021
Hegyi G, Rosivall B, Szöllősi E, Hargitai R, Eens M, Török J (2008) Phenotypic plasticity in a conspicuous female plumage trait: information content and mating patterns. Anim Behav 75:977–989. https://doi.org/10.1016/j.anbehav.2007.08.009
Hemborg C, Sanz J, Lundberg A (2001) Effects of latitude on the trade-off between reproduction and moult: a long-term study with pied flycatcher. Oecologia 129:206–212. https://doi.org/10.1007/s004420100710
Herényi M, Garamszegi LZ, Hargitai R, Hegyi G, Rosivall B, Szöllősi E, Török J (2014) Laying date and polygyny as determinants of annual reproductive success in male collared flycatchers (Ficedula albicollis): a long-term study. Naturwissenschaften 101:305–312
Hill GE (2006) Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ, Kevin J (eds) Bird Coloration, vol 2. Function and Evolution. Harvard University Press, Cambridge, pp 137–200
Igic B, D’Alba L, Shawkey MD (2018) Fifty shades of white: how white feather brightness differs among species. Sci Nat 105:18. https://doi.org/10.1007/s00114-018-1543-3
Insausti TC, Casas J (2008) The functional morphology of color changing in a spider: development of ommochrome pigment granules. J Exp Biol 211:780–789. https://doi.org/10.1242/jeb.014043
Jenkins JB, Mueller AJ, Thompson CF, Sakaluk SK, Bowers EK (2021) Female birds monitor the activity of their mates while brooding nest-bound young. Anim Cogn 24:613–628. https://doi.org/10.1007/s10071-020-01453-5
Jenni L, Winkler R (2004) The problem of molt and plumage homologies and the first plumage cycle. Condor 106:187–190. https://doi.org/10.1093/condor/106.1.187
Kötél D, Laczi M, Török J, Hegyi G (2016) Mutual ornamentation and the parental behaviour of male and female Collared Flycatchers Ficedula albicollis during incubation. Ibis 158:796–807. https://doi.org/10.1111/ibi.12389
Kovalev A, Filippov AE, Gorb SN (2014) Unzipping bird feathers. J R Soc Interface 11:20130988. https://doi.org/10.1098/rsif.2013.0988
Laczi M, Török J, Rosivall B, Hegyi G (2011) Integration of spectral reflectance across the plumage: implications for mating patterns. PLoS One 6:e23201. https://doi.org/10.1371/journal.pone.0023201
Laczi M, Hegyi G, Herényi M, Kiss D, Markó G, Nagy G, Rosivall B, Szöllősi E, Török J (2013) Integrated plumage colour variation in relation to body condition, reproductive investment and laying date in the collared flycatcher. Naturwissenschaften 100:983–991. https://doi.org/10.1007/s00114-013-1099-1
Laczi M, Kötél D, Török J, Hegyi G (2017) Mutual plumage ornamentation and biparental care: consequences for success in different environments. Behav Ecol 28:1359–1368. https://doi.org/10.1093/beheco/arx099
Laczi M, Balogh J, Nardou X, Török J, Hegyi G (2021) The meaning of purely structural colour: white plumage reflectance indicates feather condition. Ibis 163:407–416. https://doi.org/10.1111/ibi.12902
Laczi M, Jablonszky M, Markó G, Nagy G, Szabó G, Zsebők S, Török J, Hegyi G (2022) Data from: White plumage color as an honest indicator: feather macrostructure links reflectance with reproductive effort and success. Dryad Dataset. https://doi.org/10.5061/dryad.kprr4xh6b
Mahr K, Griggio M, Granatiero M, Hoi H (2012) Female attractiveness affects paternal investment: experimental evidence for male differential allocation in blue tits. Front Zool 9:14. https://doi.org/10.1186/1742-9994-9-14
Maia R, Gruson H, Endler JA, White TE (2019) pavo 2: new tools for the spectral and spatial analysis of colour in R. Methods Ecol Evol 10:1097–1107. https://doi.org/10.1111/2041-210X.13174
McGlothlin JW, Duffy DL, Henry-Freeman JL, Ketterson ED (2007) Diet quality affects an attractive white plumage pattern in dark-eyed juncos (Junco hyemalis). Behav Ecol Sociobiol 61:1391–1399. https://doi.org/10.1007/s00265-007-0370-x
Møller AP, Nielsen JT (2018) The trade-off between rapid feather growth and impaired feather quality increases risk of predation. J Ornithol 159:165–171. https://doi.org/10.1007/s10336-017-1483-2
Murphy ME (1996) Energetics and nutrition of molt. In: Carey C (ed) Avian Energetics and Nutritional Ecology. Chapman and Hall, New York, pp 158–198
Norberg RA (1977) An ecological theory on foraging time and energetics and choice of optimal food-searching method. J Anim Ecol 46:511–529
Pap PL, Vágási CI, Czirják GÁ, Barta Z (2008) Diet quality affects postnuptial molting and feather quality of the house sparrow (Passer domesticus): interaction with humoral immune function? Can J Zool 86:834–842. https://doi.org/10.1139/Z08-060
Perrins C (1979) British tits. Collins, London
Piedrahita P, Krings M, Nikolay P, Mundt N, Quezada G, Chango EM, Wagner H (2021) Integrity of and damage to wings, feather vanes and serrations in barn owls. Zoology 147:125930. https://doi.org/10.1016/j.zool.2021.125930
Pike N (2011) Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol 2:278–282. https://doi.org/10.1111/j.2041-210X.2010.00061.x
Prum RO, Torres R (2003) Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J Exp Biol 206:2409–2429. https://doi.org/10.1242/jeb.00431
Redpath S (1988) Vigilance levels in preening dunlin Calidris alpina. Ibis 130:555–557
Serra L (2001) Duration of primary moult affects primary quality in Grey Plovers Pluvialis squatarola. J Avian Biol 32:377–380. https://doi.org/10.1111/j.0908-8857.2001.320415.x
Shawkey MD, D’Alba L (2017) Interactions between colour-producing mechanisms and their effects on the integumentary colour palette. Phil Trans R Soc B 372:20160536. https://doi.org/10.1098/rstb.2016.0536
Shawkey MD, Hill GE (2005) Carotenoids need structural colours to shine. Biol Lett 1:121–124. https://doi.org/10.1098/rsbl.2004.0289
Shawkey MD, D’Alba L, Wozny J, Eliason C, Koop JA, Jia L (2011) Structural color change following hydration and dehydration of iridescent mourning dove (Zenaida macroura) feathers. Zoology 114:59–68. https://doi.org/10.1016/j.zool.2010.11.001
Siefferman L, Hill GE (2005) Male eastern bluebirds trade future ornamentation for current reproductive investment. Biol Lett 1:208–211. https://doi.org/10.1098/rsbl.2004.0274
Siikamäki P, Hovi M, Rätti O (1994) A trade-off between current reproduction and moult in the Pied Flycatcher – an experiment. Funct Ecol 8:587–593
Slagsvold T, Dale S (1996) Disappearance of female pied flycatchers in relation to breeding stage and experimentally induced molt. Ecology 77:461–471
Stuart-Fox D, Newton E, Mulder RA, D’Alba L, Shawkey MD, Igic B (2018) The microstructure of white feathers predicts their visible and near-infrared reflectance properties. PLoS One 13:e0199129. https://doi.org/10.1371/journal.pone.0199129
Sullivan TN, Wang B, Espinosa HD, Meyers MA (2017) Extreme lightweight structures: avian feathers and bones. Mater Today 20:377–391. https://doi.org/10.1016/j.mattod.2017.02.004
Swaddle JP, Witter MS (1997) The effects of molt on the flight performance, body mass, and behavior of European starlings (Sturnus vulgaris): an experimental approach. Can J Zool 75:1135–1146
Taff CC, Zimmer C, Vitousek MN (2019) Achromatic plumage brightness predicts stress resilience and social interactions in tree swallows (Tachycineta bicolor). Behav Ecol 30:733–745. https://doi.org/10.1093/beheco/arz010
Török J, Tóth L (1999) Asymmetric competition between two tit species: a reciprocal removal experiment. J Anim Ecol 68:338–345. https://doi.org/10.1046/j.1365-2656.1999.00283.x
Viblanc VA, Mathien A, Saraux C, Viera VM, Groscolas R (2011) It costs to be clean and fit: energetics of comfort behavior in breeding-fasting penguins. PLoS One 6:e21110. https://doi.org/10.1371/journal.pone.0021110
Walther BA, Clayton DH (2005) Elaborate ornaments are costly to maintain: evidence for high maintenance handicaps. Behav Ecol 16:89–95. https://doi.org/10.1093/beheco/arh135
Wiersma DS (2013) Disordered photonics. Nat Photonics 7:188–196. https://doi.org/10.1038/NPHOTON.2013.29
Woodcock EA, Rathburn MK, Ratcliffe LM (2005) Achromatic plumage reflectance, social dominance and female mate preference in black-capped chickadees (Poecile atricapillus). Ethology 111:891–900. https://doi.org/10.1111/j.1439-0310.2005.01120.x
Zanollo V, Griggio M, Robertson J, Kleindorfer S (2012) The number and coloration of white flank spots predict the strength of a cutaneous immune response in female Diamond Firetails, Stagonopleura guttata. J Ornithol 153:1233–1244. https://doi.org/10.1007/s10336-012-0855-x
Zhang F, Jiang L, Wang S (2018) Repairable cascaded slide-lock system endows bird feathers with tear-resistance and superdurability. P Natl Acad Sci USA 115:10046–10051. https://doi.org/10.1073/pnas.1808293115
Acknowledgements
We are very grateful for the reviewers for their constructive suggestions. We thank the Behavioral Ecology Group for the help in fieldwork.
Funding
Open access funding provided by Eötvös Loránd University. This work was supported by the National Research, Development and Innovation Office (NKFIH, grant number: K124443, K139992) and the Pilis Park Forestry.
Author information
Authors and Affiliations
Contributions
ML conceived the ideas and designed the methodology; MJ, GH, ML, GM, GN, GS, JT, and SZ collected the data; ML curated the data; GH and ML analyzed the data; ML led the writing of the manuscript. All authors contributed critically to the drafts and gave their final approval for publication.
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and institutional guidelines for the use of animals were followed. This research was conducted with a research permit from the regional nature conservation authority (PE-06/KTF/920–7/2018).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by K. McGraw
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laczi, M., Jablonszky, M., Markó, G. et al. White plumage color as an honest indicator: feather macrostructure links reflectance with reproductive effort and success. Behav Ecol Sociobiol 76, 125 (2022). https://doi.org/10.1007/s00265-022-03238-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-022-03238-x