Abstract
A common migratory pattern in birds is that northerly breeding populations migrate to more southerly non-breeding sites compared to southerly breeding populations (leap-frog migration). Not only do populations experience differences in migration distances, but also different environmental conditions, which may vary spatiotemporally within their annual cycles, creating distinctive selective pressures and migratory strategies. Information about such adaptations is important to understand migratory drivers and evolution of migration patterns. We use light-level geolocators and citizen science data on regional spring arrivals to compare two populations of common ringed plover Charadrius hiaticula breeding at different latitudes. We (1) describe and characterize the annual cycles and (2) test predictions regarding speed and timing of migration. The northern breeding population (NBP) wintered in Africa and the southern (SBP) mainly in Europe. The annual cycles were shifted temporally so that the NBP was always later in all stages. The SBP spent more than twice as long time in the breeding area, but there was no difference in winter. The NBP spent more time on migration in general. Spring migration speed was lower in the SBP compared to autumn speed of both populations, and there was no difference in autumn and spring speed in the NBP. We also found a larger variation in spring arrival times across years in the SBP. This suggests that a complex interaction of population specific timing and variation of breeding onset, length of breeding season, and proximity to the breeding area shape the annual cycle and migratory strategies.
Significance statement
Migration distance, climate, and the resulting composition of the annual cycle are expected to influence migration strategies and timing in birds. Testing theories regarding migration behaviours are challenging, and intraspecific comparisons over the full annual cycle are still rare. Here we compare the spatiotemporal distributions of two latitudinally separated populations of common ringed plovers using light-level geolocators. We found that there was a larger long-term variation in first arrival dates and that migration speed was slower only in spring in a temperate, short-distance migratory population, compared to an Arctic, long-distance migratory population. This suggests that a complex interaction of population specific timing and variation of breeding onset, length of breeding season and proximity to the breeding area shape the annual cycle and migratory behaviours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A migratory lifestyle is an adaptation to seasonal environments (Lack 1968), where high latitudes provide abundant resources during short summers (Alerstam et al. 2003) and lower latitudes provide sufficient resources and increased survival rates during the non-breeding season (Somveille et al. 2018; Winger et al. 2019). This is evident especially in birds, where the proportion of migratory populations and migration distances increase with increasing breeding latitude (Newton and Dale 1996; Newton 2008). It is also common that, within a species, northern breeding populations (in this paper, we will apply a northern-hemisphere perspective) winter farther south compared to southern populations. Such a migratory pattern is called leap-frog migration (Palmén 1874; Salomonsen 1955). Apart from differences in migration distance among such populations, they may also experience different conditions in their respective breeding and wintering areas in terms of seasonal length, climate conditions, food resources and predation risk (Meltofte 1996; Newton 2008; McKinnon et al. 2010). This may ultimately lead to spatiotemporal differences in energy budgets, constraints and opportunities, resulting in different organizations of the annual cycles (Wingfield 2008) and different behavioural adaptations associated with migration (Alerstam and Lindström 1990; Hedenström 2008).
Whenever migrants experience potential time constraints within the annual cycle, an expected response may be to maximize overall speed of migration to create more leeway for other activities (Alerstam and Lindström 1990; Hedenström and Alerstam 1997). Time constraints may arise, for example, if a given population experiences a narrow time window for reproduction due to climatic factors or if increased migration distance itself significantly increases the duration of migration, so that time spent on migration competes with other activities, such as breeding and moult (Hedenström and Alerstam 1997; Hedenström 2006; Newton 2011). Similarly, migration speed is expected to be higher in spring than in autumn (Alerstam and Lindström 1990) due to the time component in the fitness advantage of arriving before competitors (McNamara et al. 1998; Kokko 1999) and/or to maximize the number of breeding attempts (Morrison et al. 2019; Halupka et al. 2021).
The timing of annual events, in particular migration, may differ between populations of the same species, which experience different environmental conditions at the departure and/or destination sites. For example, latitude provides a proxy for spring onset and length of the breeding season (Baker 1939; Rötzer and Chmielewski 2001; Gow et al. 2019). In general, onset of spring occurs later and the breeding season is shorter at northerly compared to southerly latitudes. Previous studies that have characterized the timing of the annual cycles in populations breeding at different latitudes have found that populations breeding at northerly latitudes generally arrive and depart later compared to conspecifics breeding at more southerly latitudes (e.g. Conklin et al. 2010; Briedis et al. 2016) and that this pattern is consistent across the annual cycle (but see Gow et al. 2019). In general, timing of migration is endogenously controlled and set by the local photoperiod (Gwinner 1996; Åkesson et al. 2017). However, other cues such as local weather (Dänhardt and Lindström 2001) may also be used to fine tune departure and arrival decisions, especially if there is a high correlation of environmental conditions between sites (Drent et al. 2003; Bauer et al. 2020). Populations migrating shorter distances have been suggested to experience high correlations in environmental conditions between wintering and breeding areas. Thus, given inter-annual variation in the onset of spring, short-distance migratory populations are expected to adjust spring departure to match arrival in the breeding area when conditions are favourable (Alerstam and Högstedt 1980). In contrast, long-distance migratory populations may lack such a correlation between conditions at the wintering site and those in the breeding area. Theoretical work suggests that when low correlation occurs, timing of spring migration should coincide with a long-term average arrival of spring, or even be later, to reduce the risk of being too early (Bauer et al. 2020), which may have direct or indirect fitness consequences (Kokko 1999). Thus, long-distance migratory populations are expected to rely more on the endogenous migratory program, which is set by more predictable cues such as daylength (Alerstam and Högstedt 1980; Gwinner 1996). As a result, between species and for populations within species, the timing of annual events and particularly timing associated with spring migration are expected to be more variable among short-distance migratory populations compared to long-distance migratory populations.
The common ringed plover (Charadrius hiaticula, hereafter ringed plover) is a small-sized shorebird with a large latitudinal breeding range in Europe, ranging from high Arctic to temperate latitudes (Delany 2009). The ringed plover is a textbook example of a leap-frog migrant (Hale 1980; Alerstam 1990; Newton 2008). Based on ringing recoveries, the general pattern among populations breeding in Europe is that northern breeding populations, particularly those from Arctic/sub-Arctic Scandinavia and Russia, winter in West Africa, while southern breeding populations from temperate Europe winter along the European Atlantic coast and Morocco in northwest Africa (Salomonsen 1955; Taylor 1980; del Hoyo 1996). The pattern also seems to be nested within itself, so that populations breeding on the British Isles (representing the southwestern limit of the European breeding range) are mainly sedentary and breeding populations towards the north “leap-frog” their southern counterparts in subsequent order during autumn migration (Salomonsen 1955). Tracking studies of two European populations have revealed that ringed plovers from southern Sweden utilize wintering sites along the whole European Atlantic coast, including Morocco (Hedh and Hedenström 2020), and ringed plovers from northern Norway winter along the coast of West Africa (Lislevand et al. 2017). Also, tracked populations breeding in Arctic Canada and sub-Arctic Russia have been shown to winter in West and East Africa, respectively (Tomkovich et al. 2017; Léandri-Breton et al. 2019). Apart from the differences in migration distances, temperate breeding populations generally experience longer breeding seasons, with frequent reports of successive clutches within monogamous pairs, and up to 2 months earlier egg-laying dates compared to populations breeding on more northerly latitudes, particularly those breeding in the Arctic (north of the Arctic Circle) and alpine areas within the species breeding range (Väisänen 1977; Pienkowski 1984a; Blomqvist et al. 2001; Wallander and Andersson 2003).
Here we use light-level geolocator data from two populations of ringed plovers breeding in Arctic (henceforth the ‘northern breeding population’, NBP) and temperate Sweden (the ‘southern breeding population’, SBP), and citizen science data on arrival dates for the corresponding regions over 15 years, to investigate how migration distance and variations of the annual cycles within a species affect migratory behaviours. First, we ask whether the NBP winters primarily in West Africa, which based on previous published information on the SBP wintering location (Hedh and Hedenström 2020) should generate a clear leap-frog migration pattern. If so, we first predict that regional arrival dates corresponding to the breeding location for the SBP exhibit larger between year variation compared to the NBP. Second, because of the longer migration distance and more pressed time schedule, we predict that the NBP will have a higher migration speed compared to the SBP and that migration speed in general will be higher in spring than in autumn. We also discuss ecological consequences of the spatiotemporal organization of the annual cycle and migration strategy for the two populations.
Methods
Field work and data collection
Common ringed plovers were studied at Ottenby, southern Sweden (56° 13′58″ N, 16° 24′40″ N), and at Abisko, northern Sweden (68° 21′32″ N, 18° 29′02″ E), between the years 2013 (from 2014 at Abisko) and 2017. At each site, we deployed light-level geolocators (Abisko, n = 12 in 2014, n = 7 in 2015, n = 8 in 2016; Ottenby, n = 18 in 2013, n = 12 in 2014, n = 16 in 2015, n = 7 in 2016) of the model MK10s (in 2013 batch without ‘stalk’) from Migrate Technology Ltd. The geolocators were attached with a leg-loop harness, and the total mass of the device (1.6 g) constituted on average to 1.4% of the birds’ mass at capture. Geolocators were retrieved during the following years until and including 2018. In total, 24 geolocators were retrieved at Ottenby (distributed on 20 individuals) and 11 in Abisko (distributed on 8 individuals). Eight geolocators from Ottenby and 1 from Abisko contained data on 2 autumn migrations and 1 spring migration. As a result, 32 and 12 autumn tracks and 24 and 11 spring tracks from Ottenby and Abisko were available for analyses, respectively. One geolocator carried by an individual breeding at Ottenby failed on the northward migration and is therefore not included as a complete track. However, autumn departure and arrival date, as well as spring departure dates were recorded by this device and are included in the analyses.
To evaluate differences in year-to-year variation in spring arrival between the SBP and the NBP, we obtained citizen science data from the Species Observation System (http://www.artportalen.se, Swedish Species Information Centre), collected between 2005 and 2020 in the two regions in which the studied populations breed (Torne Lappmark and Öland, for the NBP and SBP, respectively). We assumed that the earliest arriving individuals in each breeding region will also breed there (Conklin et al. 2010; Beauchamp et al. 2020), and so we used the average date of the three first observations in each region as a measure of yearly variation in arrival date. We included only observations after May 10 in Torne Lappmark and February 15 on Öland, respectively, in order to avoid inclusion of potentially overwintering birds (Öland) or unusually early individuals. Doing so we omitted 2 observations in Torne Lappmark and 12 observations from Öland (of which 4 were in January and thus likely concern overwintering individuals) that were observed earlier than the pre-defined date. Only observations that were made at different localities, or were made at the same locality but ≥ 7 days apart, were used as independent observations.
Geolocator analysis
Light data were corrected for clock drift, and twilights were defined using the R package TwlGeos (Lisovski et al. 2015) using a threshold value of 5 lx. Annotated twilight events were visually inspected before proceeding, and obvious erroneous events were either corrected (based on the timing of twilight events during the pre- and proceeding days) or removed if the event was considered being caused by, e.g. artificial light in the middle of the night.
Positions for each geolocator were generated in the R package GeoLight (Lisovski and Hahn 2012). In order to find the corresponding sun angle to the set threshold value and each individual geolocator, a Hill-Ekstrom calibration of the main stationary period during the non-breeding season was used (Ekstrom 2004). This was done by modelling latitudes against several possible sun elevation angles with 0.1° increments. The sun elevation angle that minimized the error in latitude around the autumn equinox and generated stable latitudinal positions over the course of the wintering period (i.e. no systematic concave or convex shape on the latitude versus time diagram) was selected for each geolocator. The error of the loggers was 2.21° (244 km) in latitude and 1° (68 km) in longitude and was estimated from a period of time (Dec 15–Jan 15) when all but two individuals were stationary, as indicated by unchanged longitudes, and as far away in time from the equinoxes as possible to calculate mean ± SD latitude and longitude. This period of time and method was chosen because ‘roof-top’ estimation gives a less accurate estimate of latitude compared to in habitat calibration and produces a larger error (Lisovski et al. 2012), while calculating the error ‘on-board’ from a known site was not possible, since the birds breeding in Abisko (the NBP) experienced more or less 24-h day light.
Around the autumn and spring equinoxes, it was not possible to use the threshold method to determine latitude because night and day are equally long everywhere (Ekstrom 2004). After visually inspecting the data, we excluded all positions between 5 days prior and 20 days after the autumn equinox and between 20 days prior and 5 days after the spring equinox. Given the general migration period of the ringed plover, positioning during movement periods may be affected in the autumn for birds breeding in Abisko (7 out of 12) and during spring for birds breeding at Ottenby (19 out of 23) (Lislevand et al. 2017; Hedh and Hedenström 2020).
Defining stationary periods in time and space
In this study, we considered three types of stationary periods: breeding, wintering and stopovers during migration. The breeding period was defined as the time in which the birds reside within the breeding area. Winter residency was defined differently for the NBP and the SBP. As we expect birds from the NBP to migrate farther compared to SBP, we defined any stationary period in Europe regardless of duration as stopovers for the NBP, given that migration subsequently proceeded. However, any stationary period ≥ 20 days in Africa, south of Sahara, was defined as wintering. This is the minimum time required to fuel up to cover the flight distance between the mouth of Senegal River and southern Ghana (which is the most south easterly wintering site recorded in this study, see the ‘Results’ section), assuming a fuel deposition rate of 1.7%/day (see the ‘Migration distance, duration and speed’ and ‘Results’ sections). For simplicity we used the same cut-off for the SBP; however, two exceptions to this rule were made for two individuals. Both spent 36 and 20.5 days, respectively, in the German and Danish Wadden Sea before continuing to their wintering sites in western France and Western Africa. These exceptions were motivated (i) by the fact that very few ringed plovers winter in the Wadden Sea (Meltofte et al. 1994; Laursen and Frikke 2013), (ii) that the first mentioned individual subsequently continued migration to the same wintering area as in a previous year and (iii) the second individual continued to West Africa. As the temporal and spatial resolutions of light-level geolocators are relatively low, stationary periods between the breeding and wintering period had to last ≥ 3 days to count as stopovers. This arbitrary cut-off is higher than those commonly used in other studies using automatic tools to identify stationary periods (e.g. Lislevand et al. 2017; Léandri-Breton et al. 2019), but not too long in order to avoid shorter stops. We want to stress that the remaining time does not necessarily equate to in-flight time but can also include shorter stops that were not detectable.
Departure and arrival times, which define start and termination of migratory and stationary periods, were primarily identified by visually inspecting longitude plots in combination with generated maps (Machín et al. 2015; Hedh and Hedenström 2020). This approach is justified in general by the fact that (i) the spatial error is lower in the longitudinal axis compared to latitude (particularly around the equinoxes), and (ii) for the ringed plover in particular by the observations of a general southwest-northeast migratory direction in this species (based on ringing recoveries, Fransson et al. 2008), and the potential for fast movements between geographical sites due to high flight speeds (Pennycuick et al. 2013). Due to the near 24-h daylight periods around the date of departure and arrival in the NBP (August and late May–early June), it was not always possible to derive positions (this occurred when the logged light intensity did not go below the chosen threshold value) and hence define departure and arrival times. As a remedy, the light threshold value was adjusted to 550 lx, and the process of positioning was repeated. In all cases, this adjustment generated well-defined positions allowing the estimation of autumn departure of the individuals from the NBP. However, same adjustments did not generate well-defined positions of spring arrivals, because by the time of arrival the light regime was already at 24-h daylight. Therefore, we estimated individual spring arrival dates for birds of the NBP by adding 0.5 days to the last known position. This estimate corresponds to ~ 700 km travelled distance (if the air speed is 16 m/s (Pennycuick et al., 2013)), which is approximately the remaining flight distance given that most of the last detectable positions occurred over central Sweden or southern Norway.
Geographical positions for the stationary periods (except for the breeding areas, which for both populations are known) were defined by averaging the generated, bidaily positions within the above defined time periods.
Migration distance, duration and speed
Migration distances for each track were calculated as the summed great circle distance (orthodromes) between consecutive stationary positions using the Haversine formula. When it was not possible to determine positions during movement phases around the equinoxes, we calculated the orthodrome distance between the last known position and the consecutive known position. This yields a conservative estimate of total migration distance, but it is possible that distance becomes underestimated in autumn for individuals of the NBP and in spring for the SBP. However, this conservative way of solving the problem has only minor effects on the estimation of total migration distance. First, in the case of the SBP, 8 of the 19 affected individuals made a rapid movement from the wintering to the breeding ground in spring (departure and arrival were estimated by longitude, see above about timing) lasting on average 1.8 days (SD = 0.9) and covering a (orthodromic) distance of 1901 km (SD = 528). Hence, the average speed was 1056 km/day, which is only marginally slower than a direct flight at 16 m/s. Second, for 10 of the 19 affected individuals from the SBP that had stopovers between the wintering and breeding sites, we calculated the ratio between total migration distance (including at least one stopover, i.e. detour) and orthodromic distance in autumn and found that the distances only differed with an average 1.3% (SD = 1.7). Therefore, we do not find any reason to assume that the detours would be longer in spring and that the small difference will have little effect on speed calculation. Third, in the case of the NBP, in 3 out of 7 affected autumn migrations, the period for which positioning was problematic started just before or right after departure from the last stopover site. In all cases, the birds made rapid movements towards the wintering site (arrival estimated by longitude, see above about timing) lasting on average 2.2 days (SD = 0.58) and covering a (orthodromic) distance of 2793 km (SD = 723), resulting in an average speed of 1277 km/day. Again, this suggests a more or less direct flight, making the orthodrome a justifiable approximation. Finally, in 3 out of 7 flights, birds made intermediate stopovers as revealed by longitudes. Given the shape of the west African coast and assuming that ringed plovers mainly use coastal sites during autumn migration (Lislevand et al. 2017), it is possible to assign latitudes associated with the longitudes. For each individual, we generated positions during the critical period and calculated the total distances, which we compared with a direct orthodromic distance from the last known site. By this procedure, the distance increased only with 0.6% (SD = 0.8) compared with the orthodromic distance, and so we used the orthodromic distances in the analyses. Only in one individual of the NBP that first moved down the west coast of Africa (as indicated by available longitudes) and subsequently moved inland (eastwards) before the end of the critical period did we retain an estimated latitude position based on longitude.
While comparing migration duration and speeds between seasons, populations or individuals, it is important to account for all fuelling episodes, including the time allocated to fuelling before the first recorded flight (Alerstam and Lindström 1990). The logic behind this is that any difference between groups or individuals in time allocated to fuelling (depending on a multitude of factors such as food abundance, food availability and weather conditions; as reviewed in Lindström 2003) before the first flight will in turn result in differences in how much time is allocated to fuelling between the recorded departure and arrival to the final destination (i.e. the period that in this study is known from the geolocators). Hence, some groups/individuals may do longer or direct flights, whereas others do shorter flights depending on how much fuel has been put on (Piersma 1987). Thus, if such differences occur and migration duration and speed are based on the duration recorded by the geolocators only, this could result in incomparable estimates (Lindström et al. 2019). Therefore, duration of migration was defined as the period between initiation of fuelling and arrival at the final destination (Alerstam and Lindström 1990). In almost all cases (including this study), the duration of the first fuelling period is unknown and therefore has to be estimated (Lindström 2020). To estimate this period for each individual in each season and population, we divided the fuel deposition rate (FDR) at the departure sites with the fuel load needed to cover the first recorded flight distance in each season (Lindström et al. 2019). The fuel load, expressed as a fraction of the lean bod mass (LBM), was calculated using the flight range equation
where Y is the flight distance, f is the fuel load and the coefficient c is a composite factor including morphology, energy content of the fuel and energy conversion efficiency (Alerstam and Lindström 1990; Alerstam and Hedenström 1998). We assumed c = 15, 000, which is a reasonable value for a shorebird (Gudmundsson et al. 1991) and used a FDR of 1.7% of LBM as measured in ringed plovers migrating during autumn in SE Sweden (Hedh and Hedenström 2016). Estimating this period based on a single FDR can be problematic since the FDR can vary between seasons and regions. However, including a fuelling period to cover the first flight bout with a fixed FDR gives a more realistic estimate of migration duration than without it. Total migration speed for each track was subsequently calculated by dividing migration distance with migration duration.
Data analyses
To test for differences in duration at each annual cycle stage (i.e. migration, breeding and wintering), we constructed three linear mixed effect models (LMM). For breeding and wintering duration, we included population, sex and the interaction between sex and population as fixed effects and individual as random intercept. For duration of migration, we also included season as a fixed effect. To test for differences in departure and arrival times (day number, Jan 1 = 1), we constructed four LMMs, one for each event (i.e. departure from breeding site, arrival to winter site, departure from winter site and arrival at breeding site), in which population, sex and the interaction between the two were included as fixed effects, and individual as random intercept due to repeated observations. LMMs were performed using the ‘lmer’ function in the R package ‘lme4’ (Bates et al. 2015), and p-values for fixed effects were calculated using ‘lmerTest’.
We also attempted to test for differences in migration speed between populations and seasons using LMM’s, but this resulted in singular fits when including individual as random effect. Therefore, we reduced the dataset in such way that seasonal tracks from individuals that had been followed more than once in either season were excluded randomly (e.g. if one individual was tracked for two autumns and one spring season, one of the autumn tracks was excluded randomly). We then performed four Mann–Whitney-Wilcoxon tests using the ‘wilcox.test’ in the basic ‘stat’ package in R: two in which we compared autumn and spring migration speeds within population and two in which we compared the migration speed between the two populations within seasons.
To test for differences in year-to-year variation in spring arrival date between the two populations, we used the average date of the three first observations (obtained from the Swedish Species Observational System, see above) for each population to fit simple linear regressions and subsequently comparing the resultant residuals using an F-test. All statistics were carried out using R ver. 4.0.3 (R Development Core Team 2020). We did not record data blind, because our study involved focal animals carrying geolocators.
Results
Geographical patterns
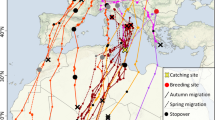
All but one individual from the SBP wintered along the European west coast and the coast of Morocco, between Ireland (53.3° N) and Morocco (30.7° N) (Fig. 1). The remaining individual wintered in West Africa (most likely Guinea Bissau, 11.3° N). All individuals from the NBP wintered in West Africa, from southern West Sahara (21.5° N) to Ghana (6.6° N), both along the coast and at inland waters in southeastern Mali, western Burkina Faso and Ghana (Fig. 1). During autumn migration, individuals from the NBP and SBP travelled on average 6660 km (SD = 765 km) and 2364 km (SD = 900 km), respectively, and during spring 6503 km (SD = 448 km) and 2188 (SD = 659 km), respectively (Table 1).
Wintering areas for individual ringed plovers (Charadrius hiaticula) breeding in Abisko (68° 21′32″ N, 18° 29′02″ E), northern Sweden (the NBP; blue points), and Ottenby (56° 13′58″ N, 16° 24′40″ E), southern Sweden (the SBP: yellow points) of individuals tracked between 2014–2017 and 2013–2017, respectively. Error bars represent the latitudinal and longitudinal errors (SD) of the average positions, and squares represent the breeding areas for the NBP and the SBP, respectively. The map is in a Mercator projection
Annual timing
In autumn, average departure time (mean ± SD) from the breeding area for individuals of the SBP was July 30 ± 7.8 days, which was about 2 weeks earlier compared to individuals of the NBP, leaving their breeding area on August 12 ± 5.2 days (Tables 1 and 2, Fig. 2). Subsequently, individuals of the SBP reached their respective wintering sites on Aug 11 ± 8.4 days. For the NBP, arrivals to the wintering area occurred 4–5 weeks later, on September 23 ± 14.7 days (Tables 1 and 2, Fig. 2). In spring individuals from the SBP departed and arrived at the breeding area on March 4 ± 9.5 days and March 9 ± 8.8 days, respectively. Individuals from the NBP departed and arrived considerably later, on May 6 ± 6.5 days and June 1 ± 6.7 days, respectively (Tables 1 and 2, Fig. 2).
Median timing of migration for individual ringed plovers breeding at Abisko (68° 21′32″ N, 18° 29′02″ E), northern Sweden (the NBP, blue boxes), and Ottenby (56° 13′58″ N, 16° 24′40″ E), southern Sweden (the SBP, yellow boxes). Boxes show the 25 and 75% quantiles and whiskers the 1.5 times inter-quantile range. Jitter shows individual data points. Asterisks indicate significance levels (*** < 0.001)
Variation in spring arrival based on citizen science data
Based on citizen science data from the geographical regions of the two study sites, spring arrival date varied more between years in the region of the SBP compared to the NBP (F15 = 3.2575, p = 0.029; Fig. 3).
Variation in spring arrival dates between common ringed plovers breeding in the region of Torne Lappmark (TL), northern Sweden (in which the NBP breeds), and the province of Öland, southern Sweden (in which the SBP breeds), based on citizen science data obtained from the Swedish Species Observation System (Swedish Species Information Centre) collected between 2005 and 2020. Data are presented as a yearly variation of spring arrival to Torne Lappmark (blue dots) and Öland (yellow dots) around the fitted regression lines (dashed lines) and b the median residuals around the fitted regression lines. Boxes show the 25 and 75% quartiles, whiskers the 1.5 times inter-quartile range. Jitter shows individual data points. Shaded area in Fig. 3a represents the confidence interval. Asterisks indicate significance levels (* < 0.05)
Annual time allocation
Migration duration (including the period of initial fuelling) did not differ between spring and autumn within the two breeding populations (Tables 1 and 2, Fig. 4a, b). However, there was an effect of population in both seasons, so that individuals from the NBP spent more time on migration in both seasons (mean duration ± SD: 54 ± 13.8 days, n = 12 and 54 ± 7.9 days, n = 11, in autumn and spring, respectively; Tables 1 and 2; Fig. 4a, b) compared to individuals from the SBP (21 ± 8.7 days, n = 32 and 23 ± 8.4 days, n = 23, in autumn and spring respectively; Tables 1 and 2, Fig. 4a, b). Individuals of the SBP spent more than twice as long time in the breeding area (136 ± 12.6 days, n = 31) compared to individuals of the NBP (60 ± 9.4 days, n = 12; Table 2, Fig. 4c). There was no big difference in length of stay at the wintering sites between the two populations (186 ± 16.6 days, n = 23 and 197 ± 20.2 days, n = 11 for the SBP and NBP, respectively; Table 2; Fig. 4d).
Median duration in a autumn and b spring migration and c breeding and d wintering site duration for ringed plovers from Abisko (68° 21′32″ N, 18° 29′02″ E), northern Sweden (the NBP; blue boxes), and Ottenby (56° 13′58″ N, 16° 24′40″ E), southern Sweden (the SBP; yellow boxes). Boxes show the 25 and 75% quartiles, whiskers the 1.5 times inter-quartile range. Jitter shows individual data points. Asterisks indicate significance levels (*** < 0.001, n.s. = not significant)
Migration speed
There was no difference in median migration speed between autumn and spring in the NBP (autumn speed, 121 km day−1, 100.7–164.0 km day−1, n = 8; spring speed, 127 km day−1, 104.9–154.2 km day−1, n = 8; Wilcoxon test, W = 27, p = 0.645; Table 1, Fig. 5); however, autumn migration speed were higher compared to spring in the SBP (autumn speed, 131 km day−1, 64.2–213.1 km day−1, n = 20; spring speed, 85 km day−1, 44.6–137 km day−1, n = 19; W = 304, p < 0.001; Table 1, Fig. 5). There was no statistical difference in migration speed in autumn between the two populations (W = 74, p = 0.7842), but during spring migration, the NBP migrated faster than the SBP (W = 145, p < 0.001).
Median autumn and spring migration speeds (km day−1) in common ringed plovers from Abisko (68° 21′32″ N, 18° 29′02″ E), northern Sweden (the NBP; blue boxes), and Ottenby ((56° 13′58″ N, 16° 24′40″ E)), southern Sweden (the SBP; yellow boxes). Boxes show the 25 and 75% quartiles, whiskers the 1.5 times inter-quartile range. Jitter shows individual data points. Asterisks indicate significance levels (*** < 0.001, n.s. = not significant)
Discussion
In this study, we investigated how different migration distances and annual schedules, dictated by different breeding and wintering latitudes and associated by climate zone as proxy, affect different behaviours associated with migration and population specific annual cycles in two populations of ringed plovers breeding in northern and southern Sweden.
Geographical patterns
Our findings corroborate the general pattern of leap-frog migration among temperate and Arctic populations of ringed plovers as previously deduced on the basis of ringing recoveries (Salomonsen 1955; Taylor 1980) and recently published information of ringed plovers tracked with light-level geolocators from northern Norway and high Canadian Arctic (Lislevand et al. 2017; Léandri-Breton et al. 2019). However, individuals from the SBP utilized wintering sites from the British Isles in the north to Morocco in the south, thus covering almost the whole described European wintering range of this population. Partly the wide latitudinal range of wintering sites of the SBP is explained by an unusual sex-specific differential migration with males wintering further south than females (Hedh and Hedenström 2020). Salomonsen (1955) suggested that the northern limit of the wintering distribution of populations breeding in the southern Baltic Sea (in which the SBP breeds) was the Iberian Peninsula. Our results imply and support the notion by Taylor (1980) that the leap-frog migration pattern does seem to be less nested than described by Salomonsen (1955). Thus, the migratory connectivity among populations is lower than expected, at least among populations breeding within temperate Europe. One possible explanation for the discrepancy between our results and the winter distribution inferred by Salomonsen (1955) could be that the wintering range has shifted northwards in the last half century due to climate change, which has been observed in many short to medium distance migratory populations (La Sorte and Thompson 2007; Visser et al. 2009; Curley et al. 2020).
Timing of migration
Individuals of the NBP departed and arrived later than those of the SBP at all annual stages. This was not unexpected due to the much later onset of the breeding at northern latitudes (particularly above the Arctic circle) and in alpine areas, compared to more southern latitudes, such as southern Sweden (Väisänen 1977; Wallander and Andersson 2003; LH and AH, unpublished data). These results are similar to findings for other species, where differences in the annual schedules between populations of the same species that breed at different latitudes have been studied. For example, individual bar-tailed godwits Limosa lapponica bauri wintering in New Zealand scheduled their spring and autumn migration in order of their Alaskan breeding latitude, starting with individuals breeding farthest to the south in both autumn and spring migration (Conklin et al. 2010). Similarly, comparing two populations of collared flycatchers Ficedula albicollis, Briedis et al. (2016) found that the annual cycles were shifted so that the southern breeding population preceded the northern population in nearly all annual events. As other authors have suggested, the pattern generally suggests that breeding latitude, or more likely, the onset of breeding season as a function of local climate in the breeding areas, drives the annual schedule in migratory populations (e.g. Conklin et al. 2010; Briedis et al. 2016; Gow et al. 2019). However, there are two important differences between our study and those of Conklin et al. (2010) and Briedis et al. (2016). First, the wintering sites of the NBP and SBP were latitudinally separated by ~ 4000 km, whereas both populations of bar-tailed godwits and collared flycatchers were wintering sympatrically at the same latitude. Second, both wintering areas of godwits and flycatchers were located far away from even the southernmost breeding area, preventing either population to use local environmental cues to predict conditions at the breeding site. The SBP (this study), on the other hand, winters relatively close to the breeding ground compared to the NBP. This raises questions regarding the relative importance of endogenous and exogenous control of timing of migration between the two populations.
We found that yearly spring arrival dates varied significantly more in the region of the SBP compared to that of the NBP. Other studies have shown that short-distance migrants tend to arrive in their breeding area with larger annual variation around the mean compared to long-distance migrants (e.g. Tryjanowski and Sparks 2002; Gunnarsson and Tómasson 2011). At least one study shows that between year variation in passage date at a stopover site in short-distance migrants is correlated with environmental conditions between wintering and breeding areas (Haest et al. 2018). Similarly, arrival dates of short-distance migratory American Kestrel Falco sparverius populations were more variable than long-distance migratory populations and closely related to spring temperatures (Powers et al. 2021). Thus, our results support the notion that the SBP, which winters relatively close to the breeding area, adjust spring arrival based on environmental cues at the wintering site, being more flexible compared to the NBP. In contrast, timing of spring migration of the NBP depends to a larger degree on the endogenous program, as suggested by Alerstam and Högstedt (1980). It remains, however, unclear what cues individuals of the SBP use at the wintering or spring stopover sites to infer environmental conditions at the breeding site. Does departure from the wintering grounds also vary or is the progression of migration adjusted en route depending on the advancement of spring (e.g. Kölzsch et al. 2015)? Currently we lack data on individuals, such as repeated tracks, which potentially could address this question.
Migration duration and speed
Overall the migration speeds were within the range of theoretical predictions (Hedenström and Alerstam 1998). We found, contrary to expectations, that the SBP migrated faster in autumn compared to spring and that the spring migration speed in the NBP was higher compared to that of the SBP. These findings are in contrast with the general observation that spring migration is generally faster compared to autumn migration (Nilsson et al. 2013). Interestingly, several recent tracking studies using light-level geolocators have found that migration speed during autumn is faster compared to spring migration (European nightjar Caprimulgus europaeus, Norevik et al. 2017; Baltic dunlin Calidris alpina schinzii, Pakanen et al. 2018; Icelandic whimbrel Numenius phaeopus islandicus, Carneiro et al. 2019). However, it should be pointed out that among these studies, different approaches of calculating the migration speed were used (see below for further discussion).
Faster migration speed during autumn migration does not necessarily need to contradict the suggestion that migratory birds experience higher time constraints in spring (cf. Kokko 1999; McNamara et al. 1998). Rather, other environmental conditions may play a major role in determining migration speed. For example, wind conditions can be less favourable in spring than in autumn for some species, as reported for Icelandic whimbrels (Alves et al. 2016), or speed could be reduced if migration is against a resource gradient. Also, it has been suggested that migrants breeding at higher latitudes may slow down spring migration to be able to predict the environmental conditions along the route in order to arrive at the optimal time to the breeding area (Pakanen et al. 2018; Bauer et al. 2020). Being on a flexible schedule may be adaptive if birds arrive with surplus energy stores that are shunted into egg formation (i.e. capital breeders), or for other breeding activities, such as territorial establishment and mate attraction (Gudmundsson et al. 1991; Hedenström 2008; Kölzsch et al. 2015, 2016). The latter may be especially useful for species or populations that arrive relatively early in the season, when inclement weather conditions are still occurring (Pakanen et al. 2018; Hedh and Hedenström 2020).
In the case of the studied ringed plover populations, the length of the first flight was generally longer in spring compared to autumn, which will ultimately increase the fuelling time if we assume that FDR is the same for both populations and seasons, and thus, reduce estimated migration speed relative to autumn migration. Additionally, the time spent fuelling for a longer flight will increase disproportionally because more fuel is needed due to aerodynamic properties; an increased fuel load will increase flight cost as a result from an increased body frontal area (and hence drag) and additional mass (Alerstam and Hedenström 1998). Interestingly, even though we assumed the FDR to be the same for the NBP and SBP, and the NBP had to prepare for a longer first flight bout, the NBP reached a higher migration speed in spring compared to the SBP. The interpretation of this observation must be that migration speed between the stopover after the first recorded flight and the breeding area (for which we have data on actual duration) is in fact higher in the NBP compared to the SBP. This could either mean that wind conditions are more favourable and thus offering more wind assistance or, but not mutually exclusive, FDRs at European stop over sites are higher later in spring when the NBP passes through. Migratory populations have most likely adapted their annual schedules to the spatiotemporal suitability of resource peaks for survival, migration and breeding (Bauer et al. 2016). For instance, Drent and Piersma (1990) suggested that the resource pulses experienced by Siberian populations of bar-tailed godwits Limosa lapponica taymyrensis, at both the African wintering grounds and subsequent European stopover sites in spring, coincide better with the migratory timing to reach the breeding areas, compared to if these populations would have wintered in Western Europe like more short-distance migratory populations. Thus, if the higher spring migration speed by the NBP at least partly is due to faster fuelling later in the season at European stopover sites, spatiotemporal differences in resource peaks can be a causal explanation of leap-frog migration in the case common ringed plover.
Organization of the annual cycle and ecological consequences of leap-frog migration
The SBP spent twice as long time in the breeding area compared to the NBP. This explains, at least temporally, why ringed plovers in temperate regions are able to lay and raise up to two successive clutches (Blomqvist et al. 2001). The most relevant gain for the SBP is probably the substantial leeway to compensate for depredated or otherwise lost clutches (Morrison et al. 2019), given the relatively high predation rate in temperate areas (Wallander and Andersson 2003). Because the ringed plover is socially monogamous, has an incubation period of around 25 days (Pienkowski 1984a), and a chick rearing period of around 20–25 days (Pienkowski 1984b), the short summer does not allow birds of the NBP to raise successive clutches. In fact, even reports of replacement clutches are rare among Arctic shorebirds and Arctic ringed plovers in particular (Pienkowski 1984a, b; Naves et al. 2008), although it may occur occasionally (LH unpubl. observations). It is also possible that temperate breeding areas provide suitable conditions for individuals to initiate and finish most of primary moult after breeding and before autumn migration (Walters 1984), which could partly explain the long period of residency in the breeding area by individuals of the SBP. However, information on the extent of moult in the breeding area of our populations is limited, although a few observations confirm moult initiation already in incubating individuals (LH unpubl. observations). Contrary, ringed plovers breeding in the arctic do not initiate moult until November, after arrival to the wintering area (Prater et al. 1977; Insley and Young 1981; Meissner et al. 2010). Moult is energetically expensive (e.g. Lindström et al. 1993), and so starting moult early may be adaptive, or even a necessity, in populations utilizing a temperate wintering range, since more energy may be needed for thermoregulation compared to African wintering grounds (Meltofte 1996; Kersten et al. 1998; Machín et al. 2018). Trade-offs between moult, breeding and migration have been shown in golden plovers Pluvialis apricaria, where Icelandic populations finish the primary moult at the breeding sites, while continental (Arctic and sub-Arctic) populations suspend moult until they reach more southerly stopover sites (Machín et al. 2018). The latter populations even show a trade-off between moult and fuelling at a southern fuelling site, in which birds are not putting on fuel stores while moulting, which further suggests that moult has a direct (energetically) or indirect (elevated predation risk) cost (Lindström et al. 2010).
Demographic parameters, such as survival, are the links between ecology and evolution, affecting life history characteristics within populations. Mortality rate is typically elevated during periods of migration compared with periods of residency (Sillett and Holmes 2002; Klaassen et al. 2014; Lok et al. 2015; Buechley et al. 2021; but see Leyrer et al. 2013). Birds of the NBP spend 30% of the annual cycle on migration, which is roughly 2.5 times longer than the SBP (12%), which means that individuals of the NBP likely suffer higher migration-related mortality than birds of the SBP. In contrast, the SBP, mainly wintering in the temperate areas in Europe along the Atlantic coast, may suffer elevated mortality rate due to more frequent cold spells and associated food shortage than the NBP. Hence, assuming annual survival is equal among all ringed plovers, mortality may be partitioned differently between stages of the annual cycle (Greenberg 1980; Buechley et al. 2021). Currently, however, we lack data on survival for the populations studied here to fully evaluate the general effect of mechanisms affecting population specific mortality at different stages across the annual cycle and migration strategies particularly.
Conclusions
We used light-level geolocators to establish population specific spatiotemporal use throughout the whole annual cycle in two populations of common ringed plovers with known breeding sites. We confirm that these two populations exhibit a leap-frog migration pattern, in which the NBP winters in Africa and the SBP mainly winters in Europe, with minor overlap between the populations. We show that despite longer migration distance and a more compressed breeding season, individuals from the NBP were only migrating faster compared to the SBP in spring. Interestingly, the NBP being faster in spring is best explained by a slower spring compared to autumn migration among individuals in the SBP, as no difference between autumn and spring migration speeds was found in the NBP. Slower migration in spring compared with autumn is rare (e.g. Nilsson et al. 2013) but is likely a consequence of low fuelling rate at northern wintering sites. Wintering at northern sites may however still be beneficial if the wintering location is close enough to the breeding location so that individuals can respond to correlated environmental cues for timing of spring migration (Alerstam and Högstedt 1980; Bauer et al. 2020). Such environmental cues can be useful to optimally time migration to the yearly variation in arrival of spring. Indeed, we showed, based on citizen science data collected over a 15-year period, that the region in which the SBP breeds exhibit larger variation in first arrival dates compared to the region in which the NBP breeds. Given the almost three times longer migration distance, higher migration speed in spring among individuals in the NBP suggests that FDRs in subsequent stopover sites (primarily in Europe) may be high and that the NBP has optimized the migration both in time and space (including the farther migration to West Africa) to seasonal resource peaks (Drent and Piersma 1990; Bauer et al. 2016). Taken together it seems like the NBP and SBP are trading-off energy and time differently during parts of the annual cycle. Yet, basic large-scale ecological data are still lacking to fully explain all patterns. Our results warrant more studies on spatiotemporal variation in migration and moult strategies across the annual cycle for geographically segregated populations of common ringed plovers, as well as individual variation in migration patterns (temporal and spatial) based on repeated tracking of individuals and demographic parameters such as seasonal survival rates.
Data availability
Data are available at https://doi.org/10.5061/dryad.5dv41ns68.
References
Åkesson S, Ilieva M, Karagicheva J, Rakhimberdiev E, Tomotani B, Helm B (2017) Timing avian long-distance migration: from internal clock mechanisms to global flights. Phil Trans R Soc B 372:20160252. https://doi.org/10.1098/rstb.2016.0252
Alerstam T (1990) Bird Migration. Cambridge University Press, Cambridge
Alerstam T, Hedenström A (1998) The development of bird migration theory. J Avian Biol 29:343–369. https://doi.org/10.2307/3677155
Alerstam T, Hedenström A, Åkesson S (2003) Long-distance migration: evolution and determinants. Oikos 103:247–260
Alerstam T, Högstedt G (1980) Spring predictability and leap-frog migration. Ornis Scand 11:196–200. https://doi.org/10.2307/3676124
Alerstam T, Lindström Å (1990) Optimal bird migration: the relative importance of time, energy, and safety. In: Gwinner E (ed) Bird Migration: the physiology and ecophysiology. Springer-Verlag, Berlin, pp 331–351
Alves JA, Dias MP, Méndez V, Katrínardóttir B, Gunnarsson T (2016) Very rapid long-distance sea crossing by a migratory bird. Sci Rep 6:38154. https://doi.org/10.1038/srep38154
Baker JR (1939) The relation between latitude and breeding seasons in birds. Proc Zool Soc Lond 108:557–582. https://doi.org/10.1111/j.1096-3642.1939.tb00042.x
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bauer S, Lisovski S, Hahn S (2016) Timing is crucial for consequences of migratory connectivity. Oikos 125:605–612. https://doi.org/10.1111/oik.02706
Bauer S, McNamara JM, Barta Z (2020) Environmental variability, reliability of information and the timing of migration. Proc R Soc B 287:20200622. https://doi.org/10.1098/rspb.2020.0622
Beauchamp AT, Guglielmo CG, Morbey YE (2020) Stopover refuelling, movement and departure decisions in the white-throated sparrow: the influence of intrinsic and extrinsic factors during spring migration. J Anim Ecol 89:2553–2566. https://doi.org/10.1111/1365-2656.13315
Blomqvist D, Wallander J, Andersson M (2001) Successive clutches and parental roles in waders: the importance of timing in multiple clutch systems. Biol J Linn Soc 74:549–555. https://doi.org/10.1006/bijl.2001.0593
Briedis M, Hahn S, Gustafsson L, Henshaw I, Träff J, Král M, Adamík P (2016) Breeding latitude leads to different temporal but not spatial organization of the annual cycle in a long-distance migrant. J Avian Biol 47:743–748. https://doi.org/10.1111/jav.01002
Buechley ER, Oppel S, Efrat R et al (2021) Differential survival throughout the full annual cycle of a migratory bird presents a life-history trade-off. J Anim Ecol 90:1228–1238. https://doi.org/10.1111/1365-2656.13449
Carneiro C, Gunnarsson TG, Alves JA (2019) Faster migration in autumn than in spring: seasonal migration patterns and non-breeding distribution of Icelandic whimbrels Numenius phaeopus islandicus. J Avian Biol 2019:e01938. https://doi.org/10.1111/jav.01938
Conklin JR, Battley PF, Potter MA, Fox JW (2010) Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat Commun 1:67. https://doi.org/10.1038/ncomms1072
Curley SR, Manne LL, Veit RR (2020) Differential winter and breeding range shifts: implications for avian migration distances. Divers Distrib 26:415–425. https://doi.org/10.1111/ddi.13036
Dänhardt J, Lindström Å (2001) Optimal departure decisions of songbirds from an experimental stopover site and the significance of weather. Anim Behav 62:235–243. https://doi.org/10.1006/anbe.2001.1749
del Hoyo J (1996) Handbook of the Birds of the World, vol. 3 Hoatzin to Auks. Lynx edicions, Barcelona
Delany S (2009) An atlas of wader populations in Africa and Western Eurasia. Wageningen, Wetlands International
Drent R, Both C, Green M, Madsen J, Piersma T (2003) Pay-offs and penalties of competing migratory schedules. Oikos 2:274–292
Drent RH, Piersma T (1990) An exploration of the energetics of leap-frog migration in Arctic breeding waders. In: Gwinner E (ed) Bird migration: the physiology and ecophysiology. Springer-Verlag, Berlin, pp 399–412
Ekstrom PA (2004) An advance in geolocation by light. Mem Natl Inst Polar Res Spec Issue 58:210–226
Fransson T, Österblom H, Hall-Karlsson S (2008) Svensk Ringmärkningsatlas, vol. 2. Bulls Graphics, Halmstad
Gow EA, Burke L, Winkler DW et al (2019) A range-wide domino effect and resetting of the annual cycle in a migratory songbird. Proc R Soc B 286:20181916. https://doi.org/10.1098/rspb.2018.1916
Greenberg R (1980) Demographic aspects of long-distance migration. In: Keast A, Morton ES (eds) Migrant birds in the neotropics - ecology, behaviour, distribution and conservation. Springer, Berlin, pp 493–504
Gudmundsson GA, Lindstrom Å, Alerstam T (1991) Optimal fat loads and long-distance flights by migrating Knots Calidris canutus, Sanderlings C. alba and Turnstones Arenaria interpres. Ibis 133:140–152
Gunnarsson TG, Tómasson G (2011) Flexibility in spring arrival of migratory birds at northern latitudes under rapid temperature changes. Bird Study 58:1–12. https://doi.org/10.1080/00063657.2010.526999
Gwinner E (1996) Circannual clocks in avian reproduction and migration. Ibis 138:47–63. https://doi.org/10.1111/j.1474-919x.1996.tb04312.x
Haest B, Hüppop O, Bairlein F (2018) The influence of weather on avian spring migration phenology: what, where and when? Glob Change Biol 24:5769–5788. https://doi.org/10.1111/gcb.14450
Hale WG (1980) Waders, 1st edn. William Collins Sons & Co Ltd, London
Halupka L, Borowiec M, Neubauer G, Halupka K (2021) Fitness consequences of longer breeding seasons of a migratory passerine under changing climatic conditions. J Anim Ecol 90:1655–1665. https://doi.org/10.1111/1365-2656.13481
Hedenström A (2006) Scaling of migration and the annual cycle of birds. Ardea 94:399–408
Hedenström A (2008) Adaptations to migration in birds: behavioural strategies, morphology and scaling effects. Phil Trans R Soc B 363:287–299. https://doi.org/10.1098/rstb.2007.2140
Hedenström A, Alerstam T (1997) Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J Theor Biol 189:227–234. https://doi.org/10.1006/jtbi.1997.0505
Hedenström A, Alerstam T (1998) How fast can birds migrate? J Avian Biol 29:424–432
Hedh L, Hedenström A (2016) Autumn migration strategies and trapping numbers in the common ringed plover Charadrius hiaticula in Southern Sweden. Ardea 104:227–237. https://doi.org/10.5253/arde.v104i3.a3
Hedh L, Hedenström A (2020) The migration pattern of a monogamous shorebird challenges existing hypotheses explaining the evolution of differential migration. J Theor Biol 487:110111. https://doi.org/10.1016/j.jtbi.2019.110111
Insley H, Young L (1981) Autumn passage of ringed plovers through Southampton water. Ringing Migr 3:157–164. https://doi.org/10.1080/03078698.1981.9673774
Kersten M, Bruinzeel L, Wiersma P, Piersma T (1998) Reduced basal metabolic rate of migratory waders wintering in coastal Africa. Ardea 86:71–80
Klaassen RHG, Hake M, Strandberg R, Koks BJ, Trierweiler C, Exo K-M, Bairlein F, Alerstam T (2014) When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J Anim Ecol 83:176–184. https://doi.org/10.1111/1365-2656.12135
Kokko H (1999) Competition for early arrival in migratory birds. J Anim Ecol 68:940–950
Kölzsch A, Bauer S, de Boer R, Griffin L, Cabot D, Exo K-M, van der Jeugd HP, Nolet BA (2015) Forecasting spring from afar? Timing of migration and predictability of phenology along different migration routes of an avian herbivore. J Anim Ecol 84:272–283. https://doi.org/10.1111/1365-2656.12281
Kölzsch A, Müskens GJDM, Kruckenberg H, Glazov P, Weinzierl R, Nolet BA, Wikelski M (2016) Towards a new understanding of migration timing: slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos 125:1496–1507. https://doi.org/10.1111/oik.03121
La Sorte FA, Thompson FR (2007) Poleward shifts in winter ranges of North American birds. Ecology 88:1803–1812. https://doi.org/10.1890/06-1072.1
Laursen K, Frikke J (2013) Staging waterbirds in the Danish Wadden Sea 1980–2010. Dansk Orn Tidskr 107:1–184
Léandri-Breton DJ, Lamarre JF, Bêty J (2019) Seasonal variation in migration strategies used to cross ecological barriers in a nearctic migrant wintering in Africa. J Avian Biol 2019:e02101. https://doi.org/10.1111/jav.02101
Leyrer J, Lok T, Brugge M (2013) Mortality within the annual cycle : seasonal survival patterns in Afro-Siberian Red Knots Calidris canutus canutus. J Ornithol 154:933–943. https://doi.org/10.1007/s10336-013-0959-y
Lindström Å (2003) Fuel deposition rates in migrating birds: causes, constraints and consequences. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian Migration. Springer-Verlag, Berlin, pp 307–320
Lindström Å (2020) Migration tracks of waders: avoiding the pitfalls of speed estimates and inferred strategies. Wader Study 127:2–3. https://doi.org/10.18194/ws.00181
Lindström Å, Alerstam T, Hedenström A (2019) Faster fuelling is the key to faster migration. Nat Clim Change 9:288–289. https://doi.org/10.1038/s41558-019-0443-7
Lindström Å, Dänhardt J, Green M, Klaassen RHG, Olsson P (2010) Can intensively farmed arable land be favourable for birds during migration? The case of the Eurasian golden plover Pluvialis apricaria. J Avian Biol 41:154–162. https://doi.org/10.1111/j.1600-048X.2009.04810.x
Lindström Å, Visser GH, Daan S (1993) The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol Zool 66:490–510. https://doi.org/10.1086/physzool.66.4.30163805
Lislevand T, Briedis M, Heggøy O, Hahn S (2017) Seasonal migration strategies of common ringed plovers Charadrius hiaticula. Ibis 159:225–229. https://doi.org/10.1111/ibi.12424
Lisovski S, Hahn S (2012) GeoLight - processing and analysing light-based geolocator data in R. Methods Ecol Evol 3:1055–1059. https://doi.org/10.1111/j.2041-210X.2012.00248.x
Lisovski S, Hewson CM, Klaassen RHG, Korner-Nievergelt F, Kristensen MW, Hahn S (2012) Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol Evol 3:603–612. https://doi.org/10.1111/j.2041-210X.2012.00185.x
Lisovski S, Sumner MD, Wotherspoon SJ (2015) TwGeos: basic data processing for light based geolocation archival tags, https://github.com/slisovski/TwGeos
Lok T, Overdijk O, Piersma T (2015) The cost of migration: Spoonbills suffer higher mortality during trans-Saharan spring migrations only. Biol Lett 11:20140944. https://doi.org/10.1098/rsbl.2014.0944
Machín P, Fernández-Elipe J, Flores M, Fox JW, Aguirre JI, Klaassen RHG (2015) Individual migration patterns of eurasian golden plovers Pluvialis apricaria breeding in Swedish Lapland; examples of cold spell-induced winter movements. J Avian Biol 46:634–642. https://doi.org/10.1111/jav.00768
Machín P, Remisiewicz M, Fernández-Elipe J, Jukema J, Klaassen RHG (2018) Conditions at the breeding grounds and migration strategy shape different moult patterns of two populations of Eurasian golden plover Pluvialis apricaria. J Avian Biol 2018:e01709. https://doi.org/10.1111/jav.01709
McKinnon L, Smith PA, Nol E, Martin JL, Doyle FI, Abraham KF, Gilchrist HG, Morrison RIG, Bêty J (2010) Lower predation risk for migratory birds at high latitudes. Science 327:326–327. https://doi.org/10.1126/science.1183010
McNamara JM, Welham RK, Houston AI (1998) The timing of migration within the context of an annual routine. J Avian Biol 29:416–423. https://doi.org/10.2307/3677160
Meissner W, Chylarecki P, Skakuj M (2010) Ageing and sexing the ringed plover Charadrius hiaticula. Wader Study Gr Bull 117:99–102
Meltofte H (1996) Are African wintering waders really forced south by competition from northerly wintering conspecifics? Benefits and constraints of northern versus southern wintering and breeding in waders. Ardea 84:31–44
Meltofte H, Blew J, Frikke J, Rösner H-U, Smit CJ (1994) Numbers and distribution of waterbirds in the Wadden Sea. Results and evaluation of 36 simulateous counts in the Dutch-German-Danish Wadden Sea 1980–1991. IWRB Publ 34/Wader Study Gr Bull 74 Spec issue
Morrison CA, Alves JA, Gunnarsson TG, Böðvar Þ, Gill JA, (2019) Why do earlier-arriving migratory birds have better breeding success? Ecol Evol 9:8856–8864. https://doi.org/10.1002/ece3.5441
Naves L, Lanctot R, Taylor A, Coutsoubos N (2008) How often do Arctic shorebirds lay replacement clutches? Wader Study Gr Bull 115:2–9
Newton I (2008) The migration ecology of birds. Academic Press, London
Newton I (2011) Migration within the annual cycle: species, sex and age differences. J Ornithol 152:169–185. https://doi.org/10.1007/s10336-011-0689-y
Newton I, Dale L (1996) Relationship between migration and latitude among West European birds. J Anim Ecol 65:137–146. https://doi.org/10.2307/5716
Nilsson C, Klaassen RHG, Alerstam T (2013) Differences in speed and duration of bird migration between spring and autumn. Am Nat 181:837–845. https://doi.org/10.1086/670335
Norevik G, Åkesson S, Hedenström A (2017) Migration strategies and annual space-use in an Afro-Palaearctic aerial insectivore – the European nightjar Caprimulgus europaeus. J Avian Biol 48:738–747. https://doi.org/10.1111/jav.01071
Pakanen VM, Jaakkonen T, Saarinen J et al (2018) Migration strategies of the Baltic dunlin: rapid jump migration in the autumn but slower skipping type spring migration. J Avian Biol 2018:e01513. https://doi.org/10.1111/jav.01513
Palmén JA (1874) Om foglarnes flyttningsvägar. Frenckell, Helsinki
Pennycuick CJ, Åkesson S, Hedenström A (2013) Air speeds of migrating birds observed by ornithodolite and compared with predictions from flight theory. J R Soc Interface 10(20130419):20130419
Pienkowski MW (1984a) Breeding biology and population dynamics of ringed plovers Charadrius hiaticula in Britain and Greenland : nest- predation as a possible factor limiting distribution and timing of breeding. J Zool 202:83–114. https://doi.org/10.1111/j.1469-7998.1984.tb04289.x
Pienkowski MW (1984b) Behaviour of young ringed plovers Charadrius hiaticula and its relationship to growth and survival to reproductive age. Ibis 126:133–155
Piersma T (1987) Hop, skip or jump? Constrains on migration of arctic waders by feeding, fattening and flight speed. Limosa 60:185–194
Powers BF, Winiarski JM, Requena-Mullor JM, Heath JA (2021) Intra-specific variation in migration phenology of American kestrels (Falco sparverius) in response to spring temperatures. Ibis 163:1448–1456. https://doi.org/10.1111/ibi.12953
Prater AJ, Marchant JH, Vuorinen J (1977) Guide to identification and ageing of Holarctic waders. British Trust for Ornithology, Herts
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Rötzer T, Chmielewski FM (2001) Phenological Maps of Europe Clim Res 18:249–257. https://doi.org/10.3354/cr018249
Salomonsen F (1955) The evolutionary significance of bird migration. Dan Biol Medd 22:62
Sillett TS, Holmes RT (2002) Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol 71:296–308
Somveille M, Rodrigues ASL, Manica A (2018) Energy efficiency drives the global seasonal distribution of birds. Nat Ecol Evol 2:962–969. https://doi.org/10.1038/s41559-018-0556-9
Taylor RC (1980) Migration of the ringed plover Charadrius hiaticula. Ornis Scand 11:30–42. https://doi.org/10.2307/3676263
Tomkovich PS, Porter R, Loktionov EY, Syroechkovskiy EE (2017) Transcontinental pathways and seasonal movements of an Asian migrant, the Common Ringed Plover Charadrius hiaticula tundrae. Wader Study 124:175–184
Tryjanowski P, Sparks TIM (2002) Earlier arrival of some farmland migrants in western Poland. Ibis 144:62–68. https://doi.org/10.1046/j.0019-1019.2001.00022.x
Väisänen RA (1977) Geographic variation in timing of breeding and egg size in eight European species of waders. Ann Zool Fenn 14:1–25
Visser ME, Perdeck AC, van Balen JH, Both C (2009) Climate change leads to decreasing bird migration distances. Glob Change Biol 15:1859–1865. https://doi.org/10.1111/j.1365-2486.2009.01865.x
Wallander J, Andersson M (2003) Reproductive tactics of the ringed plover Charadrius hiaticula. J Avian Biol 34:259–266. https://doi.org/10.1034/j.1600-048X.2003.03109.x
Walters J (1984) The onset of primary moult in breeding Charadrius plovers. Bird Study 31:43–48. https://doi.org/10.1080/00063658409476814
Winger BM, Auteri GG, Pegan TM, Weeks BC (2019) A long winter for the Red Queen : rethinking the evolution of seasonal migration. Biol Rev 94:737–752. https://doi.org/10.1111/brv.12476
Wingfield JC (2008) Organization of vertebrate annual cycles: implications for control mechanisms. Phil Trans R Soc B 363:425–441. https://doi.org/10.1098/rstb.2007.2149
Acknowledgements
We are grateful staff from Ottenby Bird Observatory throughout the project years for help with logger deployment. We are also grateful to Ulf Molau for the help with infrastructure in Abisko. We are grateful to two anonymous reviewers for their valuable comments that have improved this manuscript substantially. This is report no 322 from Ottenby Bird Observatory.
Funding
Open access funding provided by Lund University. The study was financed by support from the Swedish Research Council (621–2012-3585, 2016–03625) to AH. This is a report from the Centre for Animal Movement Research (CAnMove) funded by a Linnaeus grant from the Swedish Research Council (349–2007-8690) and Lund University. Additional funding were received from BirdLife Sweden to LH, and Alvin's and Helge Ax:son Johnson's Foundations to LH and JD.
Author information
Authors and Affiliations
Contributions
LH, JD and AH conceived the ideas; LH, JD and AH collected the data; LH did the formal analysis of the data and drafted the manuscript with input from JD and AH. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Ethics approval
The tagging of common ringed plovers at both Abisko and Ottenby was approved by the Malmö-Lund djurförsöksetiska nämnd (permits reference numbers M33/13, M72/15). All ringing was conducted under licenses from the Swedish Ringing Office, issued to LH. All international, national and/or institutional guidelines for the use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by W. Wiltschko
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hedh, L., Dänhardt, J. & Hedenström, A. Population specific annual cycles and migration strategies in a leap-frog migrant. Behav Ecol Sociobiol 76, 2 (2022). https://doi.org/10.1007/s00265-021-03116-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03116-y