Abstract
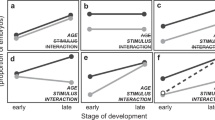
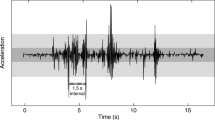
As animals develop, their abilities and needs change, altering cost/benefit trade-offs and optimal behavior responses to cues. We tested if ontogenetic changes in information use and environmentally cued hatching decisions of embryos match adaptive predictions, using red-eyed treefrogs, Agalychnis callidryas. These arboreal embryos hatch rapidly and prematurely to escape egg predators, cued by physical disturbance in attacks. Hatching early increases tadpole mortality, and premature embryos use multiple frequency and temporal properties of vibrations to assess risk, avoiding false alarms in benign disturbances such as rain. Because development increases hatchling survival, we hypothesized embryos approaching spontaneous hatching would reduce vibration sampling to reduce egg-predation risk, accepting more false alarms if cues are time-consuming to assess. We designed stimuli to elicit high or low hatching of younger embryos, with the property that reduces hatching either rapidly evident (frequency spectrum or fast temporal pattern) or time-consuming to assess (slow temporal pattern). We developed a new egg-tray vibration playback system to present motion-only cues with minimal disturbance in setup, then used it to compare responses of younger and previously untestable older embryos. The playback duration embryos experienced before or without hatching decreased with age, suggesting reduced vibration sampling. At both stages, hatching responses differed between frequencies, and among temporal patterns; nearly full-term embryos still showed selective hatching responses to cues. Hatching increased developmentally in a stimulus-dependent manner, with the greatest change in response to the slow pattern, as predicted. Developmental changes in discrimination appear well-matched to changing trade-offs, consistent with ontogenetic adaptation of embryo behavior.
Significance statement
Animal behavior changes ontogenetically for many reasons, including developing capacities to sense and assess cues and perform actions, adaptive responses to changing costs and benefits, and learning. Hatching is a critical behavior, subject to selection from pre- and post-hatching environments, that is performed during a period of rapid development. Environmentally cued hatching is widespread, and many embryos use vibrations to inform behavior. The egg-tray system we developed enables vibration playbacks to red-eyed treefrog embryos across a broader range of stages than previously possible, facilitating tests of how and why development changes behavior. Playbacks revealed that embryos at different stages assess cues and make decisions differently, with stage-specific responses matching adaptive predictions. This reveals a new level of complexity in embryo information use, motivating and enabling further research assessing the role of adaptive ontogeny and developmental constraint in early behavioral development.





Similar content being viewed by others
References
Bate M (1999) Development of motor behaviour. Curr Opin Neurobiol 9:670–675
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication. Sinauer, Sunderland
Brown RM, Iskandar DT (2000) Nest site selection, larval hatching, and advertisement calls of Rana arathooni from southwestern Sulawesi (Celebes) Island, Indonesia. J Herpetol 34:404–413
Buckley CR, Michael SF, Irschick DJ (2005) Early hatching decreases jumping performance in a direct-developing frog, Eleutherodactylus coqui. Funct Ecol 19:67–72
Caldwell MS, McDaniel JG, Warkentin KM (2009) Frequency information in the vibration-cued escape hatching of red-eyed treefrogs. J Exp Biol 212:566–575
Caldwell MS, McDaniel JG, Warkentin KM (2010) Is it safe? Red-eyed treefrog embryos assessing predation risk use two features of rain vibrations to avoid false alarms. Anim Behav 79:255–260
Cohen KL, Seid MA, Warkentin KM (2016) How embryos escape from danger: the mechanism of rapid, plastic hatching in red-eyed treefrogs. J Exp Biol 219:1875–1883
Colombelli-Negrel D, Hauber ME, Robertson J, Sulloway FJ, Hoi H, Griggio M, Kleindorfer S (2012) Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Curr Biol 22:2155–2160
Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193
Darmaillacq AS, Lesimple C, Dickel L (2008) Embryonic visual learning in the cuttlefish, Sepia officinalis. Anim Behav 76:131–134
Doody JS, Paull P (2013) Hitting the ground running: environmentally cued hatching in a lizard. Copeia 2013:159–164
Doody JS, Stewart B, Camacho C, Christian K (2012) Good vibrations? Sibling embryos expedite hatching in a turtle. Anim Behav 83:645–651
Dugatkin LA, Godin J-GJ (1992) Prey approaching predators: a cost-benefit perspective. Ann Zool Fenn 29:233–252
Endo J, Takanashi T, Mukai H, Numata H (2019) Egg-cracking vibration as a cue for stink bug siblings to synchronize hatching. Curr Biol 29:1–6
Ferrari MCO, Chivers DP (2009) Latent inhibition of predator recognition by embryonic amphibians. Biol Lett 5:160–162
FitzGibbon CD (1994) The costs and benefits of predator inspection behaviour in Thompson’s gazelles. Behav Ecol Sociobiol 34:139–148
Gomez-Mestre I, Warkentin KM (2007) To hatch and hatch not: similar selective trade-offs but different responses to egg predators in two closely related, syntopic treefrogs. Oecologia 153:197–206
Gomez-Mestre I, Wiens JJ, Warkentin KM (2008) Evolution of adaptive plasticity: risk-sensitive hatching in Neotropical leaf-breeding treefrogs. Ecol Monogr 78:205–224
Griem JN, Martin KLM (2000) Wave action: the environmental trigger for hatching in the California grunion Leuresthes tenuis (Teleostei: Atherinopsidae). Mar Biol 137:177–181
Guibe M, Poirel N, Houde O, Dickel L (2012) Food imprinting and visual generalization in embryos and newly hatched cuttlefish, Sepia officinalis. Anim Behav 84:213–217
Hall WG, Oppenheim RW (1987) Developmental psychobiology: prenatal, perinatal, and early postnatal aspects of behavioral development. Annu Rev Psychol 38:91–128
Harshaw C, Lickliter R (2011) Biased embryos: prenatal experience alters the postnatal malleability of auditory preferences in bobwhite quail. Dev Psychobiol 53:291–302
Kempster RM, Hart NS, Collin SP (2013) Survival of the stillest: predator avoidance in shark embryos. PLoS One 8:e52551
Martin K, Bailey K, Moravek C, Carlson K (2011) Taking the plunge: California grunion embryos emerge rapidly with environmentally cued hatching (ECH). Integr Comp Biol 51:26–37
Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP (2008) Learning by embryos and the ghost of predation future. Proc R Soc Lond B 275:2603–2607
Mukai H, Hironaka M, Tojo S, Nomakuchi S (2012) Maternal vibration induces synchronous hatching in a subsocial burrower bug. Anim Behav 84:1443–1448
Mukai H, Hironaka M, Tojo S, Nomakuchi S (2014) Maternal vibration: an important cue for embryo hatching in a subsocial shield bug. PLoS One 9:e87932
Nishide Y, Tanaka S (2016) Desert locust, Shistocerca gregaria, eggs hatch in synchrony in a mass but not when separated. Behav Ecol Sociobiol 70:1507–1515
Oyarzun FX, Strathmann RR (2011) Plasticity of hatching and duration of planktonic development in marine invertebrates. Integr Comp Biol 51:81–90
Romagny S, Darmaillacq AS, Guibe M, Bellanger C, Dickel L (2012) Feel, smell and see in an egg: emergence of perception and learning in an immature invertebrate, the cuttlefish embryo. J Exp Biol 215:4125–4130
Savage JM (2002) The amphibians and reptiles of Costa Rica. University of Chicago Press, Chicago
Schneider CA, Rasband WS, Elicieri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Strathmann RR, Strathmann MF, Ruiz-Jones G, Hadfield MG (2010) Effect of plasticity in hatching on duration as a precompetent swimming larva in the nudibranch Phestilla sibogae. Invertebr Biol 129:309–318
Touchon JC, Vonesh JR (2016) Variation in abundance and efficacy of tadpole predators in a Neotropical pond community. J Herpetol 50:113–119
Touchon JC, McCoy MW, Vonesh JR, Warkentin KM (2013) Effects of plastic hatching timing carry over through metamorphosis in red-eyed treefrogs. Ecology 94:850–860
Warkentin KM (1995) Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc Natl Acad Sci U S A 92:3507–3510
Warkentin KM (1999) The development of behavioral defenses: a mechanistic analysis of vulnerability in red-eyed tree frog hatchlings. Behav Ecol 10:251–262
Warkentin KM (2000) Wasp predation and wasp-induced hatching of red-eyed treefrog eggs. Anim Behav 60:503–510
Warkentin KM (2005) How do embryos assess risk? Vibrational cues in predator-induced hatching of red-eyed treefrogs. Anim Behav 70:59–71
Warkentin KM (2011a) Environmentally cued hatching across taxa: embryos respond to risk and opportunity. Integr Comp Biol 51:14–25
Warkentin KM (2011b) Plasticity of hatching in amphibians: evolution, trade-offs, cues and mechanisms. Integr Comp Biol 51:111–127
Warkentin KM, Caldwell MS (2009) Assessing risk: embryos, information, and escape hatching. In: Dukas R, Ratcliffe JM (eds) Cognitive ecology II. University of Chicago Press, Chicago, pp 177–200
Warkentin KM, Buckley CR, Metcalf KA (2006a) Development of red-eyed treefrog eggs affects efficiency and choices of egg-foraging wasps. Anim Behav 71:417–425
Warkentin KM, Caldwell MS, McDaniel JG (2006b) Temporal pattern cues in vibrational risk assessment by red-eyed treefrog embryos, Agalychnis callidryas. J Exp Biol 209:1376–1384
Warkentin KM, Caldwell MS, Siok TD, D’Amato AT, McDaniel JG (2007) Flexible information sampling in vibrational assessment of predation risk by red-eyed treefrog embryos. J Exp Biol 210:614–619
Warkentin KM, Cuccaro Diaz J, Güell BA, Jung J, Kim SJ, Cohen KL (2017) Developmental onset of escape-hatching responses in red-eyed treefrogs depends on cue type. Anim Behav 129:103–112
Whittington ID, Kearn GC (1988) Rapid hatching of mechanically-disturbed eggs of the monogenean gill parasite Diclidophora luscae, with observations on sedimentation of egg bundles. Int J Parasitol 18:847–852
Whittington ID, Kearn GC (2011) Hatching strategies in monogenean (platyhelminth) parasites that facilitate host infection. Integr Comp Biol 51:91–99
Wiedenmayer CP (2009) Plasticity of defensive behavior and fear in early development. Neurosci Biobehav Rev 33:432–441
Willink B, Palmer MS, Landberg T, Vonesh JR, Warkentin KM (2014) Environmental context shapes immediate and cumulative costs of risk-induced early hatching. Evol Ecol 28:103–116
Acknowledgments
We thank David Campbell of the Boston University Engineering Products Innovation Center for building 12 iterations of egg-holding devices and 6 iterations of attachment systems over 3.5 years. Daniel Gorelick helped construct egg-holding devices 1 year. Andrew Quitmeyer introduced us to Thermomorph, facilitating device alterations in the field. Justin Touchon consulted on statistics. Nora Moskowitz, Alina Chaiyasarikul, Karina Escobar, María José Salazar Nicholls, and Crystal Tippet helped with the egg collection and care. Maria Camila Bastos Riascos and Adeel Bhatti measured hatchling photographs. We thank the Gamboa Frog Group for discussions of the research and the Egg Science Reading Group at BU and six reviewers for comments on earlier versions of the manuscript.
Funding
This research was funded by the National Science Foundation (IOS–1354072 to KMW and JGM), Boston University, and the Smithsonian Tropical Research Institute. LARS visit to STRI was supported by the Universidad del Magdalena (Academic Arrangement 014–2015).
Author information
Authors and Affiliations
Contributions
KMW and JGM developed the egg-tray playback system. KMW, JJ, and JGM designed the experiments. JJ and KMW prepared the stimuli. KMW and LARS performed the first experiment. JJ performed the second experiment. KMW and JJ analyzed the data. KMW wrote the paper and all authors reviewed the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research was approved by the Institutional Animal Care and Use Committees of the Smithsonian Tropical Research Institute (2014-0601-2017-A3) and Boston University (14-008) and conducted under permits from the Panamanian Environmental Ministry (SE/A-46-15 and SE/A-59-16).
Additional information
Communicated by A. T. Baugh
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Movie captions (PDF 92.1 kb)
ESM 2
High-speed video of vibration playback of Stimulus 1 (MP4 811 kb)
ESM 3
Real-time video of vibration playback of Stimulus 1 to younger eggs (MP4 6015 kb)
ESM 4
Spectra and waveforms of stimulus files and recordings of playbacks from egg trays (PDF 776 kb)
ESM 5
Real-time video of vibration playback of Stimulus 2 to older eggs (MP4 3938 kb)
ESM 6
Data from egg-tray playback Experiment 1 and Experiment 2 (XLSX 37 kb)
Rights and permissions
About this article
Cite this article
Warkentin, K.M., Jung, J., Rueda Solano, L.A. et al. Ontogeny of escape-hatching decisions: vibrational cue use changes as predicted from costs of sampling and false alarms. Behav Ecol Sociobiol 73, 51 (2019). https://doi.org/10.1007/s00265-019-2663-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2663-2