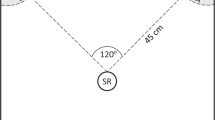
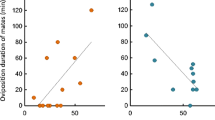
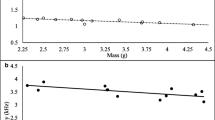
Abstract
Nocturnal light levels vary throughout the course of the lunar cycle, being darkest during the new moon and brightest during the full moon. Many nocturnal animals change their behavior in response to this natural variation in moonlight intensity. Frequently, these behavioral changes can be attributed to the way in which moonlight affects the ability of predators to spot potential prey. Mate sampling females may expose themselves to predators, making mate choice a behavior likely influenced by moonlight. Because mate choice is an important cause of sexual selection, understanding the causes of variation in mate choice decisions can yield a better understanding of the strength and direction of sexual selection under natural conditions. We predicted that female eastern gray treefrogs (Hyla versicolor) would prefer longer calls (i.e., more attractive males) and/or be choosier, under darker conditions, because cover of darkness may aid in predator evasion. However, light treatment did not affect how females responded to variation in call duration, nor did it affect female choosiness or aspects of their approach behavior. This suggests that in gray treefrogs, variation in light levels associated with the changing phases of the moon does not alter the sexual selection regime on male call traits.
Significance statement
Looking for the perfect mate can be very dangerous, especially when environmental conditions make it more likely to be spotted by potential predators. Changes in mate choice behavior associated with predator exposure is quite common in nature, yet have rarely been examined in connection with the drastic variation in nocturnal illumination associated with the changing phases of the moon. We investigated whether females of a nocturnal treefrog change their behavior depending on whether they look for mates under simulated new moon or full moon conditions. We found that females preferred longer calls under both conditions, and that they also did not move more stealthily during bright compared to dark conditions. Our results suggest that males with long calls always have a mating advantage, and that sexual selection by female choice is uniformly strong across the lunar cycle.




Similar content being viewed by others
References
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Backwell PR, Passmore NI (1990) Suitable approach perches affect female phonotaxis in an arboreal frog. Herpetologica 46:11–14
Baugh AT, Ryan MJ (2010) Ambient light alters temporal updating behaviour during mate choice in a Neotropical frog. Can J Zool 88:448–453
Bonachea LA, Ryan MJ (2011a) Predation risk increases permissiveness for heterospecific advertisement calls in túngara frogs, Physalaemus pustulosus. Anim Behav 82:347–352
Bonachea LA, Ryan MJ (2011b) Simulated predation risk influences female choice in túngara frogs, Physalaemus pustulosus. Ethology 117:400–407
Bonachea LA, Ryan MJ (2011c) Localization error and search costs during mate choice in túngara frogs, Physalaemus pustulosus. Ethology 117:56–62
Bosch J, Rand AS, Ryan MJ (2000) Signal variation and call preferences for whine frequency in the túngara frog, Physalaemus pustulosus. Behav Ecol Sociobiol 49:62–66
Buchanan BW (1993) Effects of enhanced lighting on the behaviour of nocturnal frogs. Anim Behav 45:893–899
Buchanan BW (1998) Low-illumination prey detection by squirrel treefrogs. J Herpetol 32:270–274
Campbell SR, Mackessy SP, Clarke JA (2008) Microhabitat use by brown treesnakes (Boiga irregularis): effects of moonlight and prey. J Herpetol 42:246–250
Channing A (2001) Amphibians of central and southern Africa. Comstock Publishing Associates, Ithaca, NY
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates Inc., Hilldale, NJ
Coleman SW, Patricelli GL, Borgia G (2004) Variable female preferences drive complex male displays. Nature 428:742–745
Cummings ME, Bernal XE, Reynaga R, Rand AS, Ryan MJ (2008) Visual sensitivity to a conspicuous male cue varies by reproductive state in Physalaemus pustulosus females. J Exp Biol 211:1203–1210
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London
Diekamp B, Gerhardt HC (1995) Selective phonotaxis to advertisement calls in the gray treefrog Hyla versicolor: behavioral experiments and neurophysiological correlates. J Comp Physiol A 177:173–190
Forsgren E (1992) Predation risk affects mate choice in a gobiid fish. Am Nat 140:1041–1049
Fowler-Finn KD, Rodríguez RL (2012) Experience-zediated plasticity in mate preferences: mating assurance in a variable environment. Evolution 66:459–468
Gerhardt HC (1987) Evolutionary and neurobiological implications of selective phonotaxis in the green treefrog, Hyla cinerea. Anim Behav 35:1479–1489
Gerhardt HC (1992) Conducting playback experiments and interpreting their results. In: McGregor PK (ed) Playback and studies of animal communication. Springer, New York, pp 59–77
Gerhardt HC (2005) Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution 59:395–408
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press, Chicago
Gerhardt HC, Tanner SD, Corrigan CM, Walton HC (2000) Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav Ecol 11:663–669
Godin J-GJ, Briggs SE (1996) Female mate choice under predation risk in the guppy. Anim Behav 51:117–130
Gomez D, Richardson C, Lengagne T, Plenet S, Joly P, Léna JP, Théry M (2009) The role of nocturnal vision in mate choice: females prefer conspicuous males in the European tree frog (Hyla arborea). Proc R Soc Lond B 276:2351–2358
Gong A, Gibson RM (1996) Reversal of female preference after visual exposure to a predator in the guppy, Poecilia reticulata. Anim Behav 52:1007–1015
Grant R, Halliday T, Chadwick E (2012) Amphibians’ response to the lunar synodic cycle—a review of current knowledge, recommendations, and implications for conservation. Behav Ecol 24:53–62
Hedrick AV, Dill LM (1993) Mate choice by female crickets is influenced by predation risk. Anim Behav 46:193–196
Höbel G, Barta T (2014) Adaptive plasticity in calling site selection in grey treefrogs (Hyla versicolor). Behaviour 151:741–754
Hunt J, Brooks R, Jennions MD (2005) Female mate choice as a condition-dependent life-history trait. Am Nat 166:79–92
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev 72:283–327
Johnson JB, Basolo AL (2003) Predator exposure alters female mate choice in the green swordtail. Behav Ecol 14:619–625
Kilmer JT, Fowler-Finn KD, Gray DA, Höbel G, Rebar D, Reichert MS, Rodríguez RL (2017) Describing mate preference functions and other function-valued traits. J Evol Biol 30:1658–1673
Kotler BP, Brown J, Mukherjee S, Berger-Tal O, Bouskila A (2010) Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging. Proc R Soc Lond B 277:1469–1474
Lillywhite HB, Brischoux F (2012) Is it better in the moonlight? Nocturnal activity of insular cottonmouth snakes increases with lunar light levels. J Zool 286:194–199
Longland WS, Price MV (1991) Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology 72:2261–2273
Lynch KS, Rand AS, Ryan MJ, Wilczynski W (2005) Plasticity in female mate choice associated with changing reproductive states. Anim Behav 69:689–699
Meyer K, Kirkpatrick M (2005) Up hill, down dale: quantitative genetics of curvaceous traits. Philos T Roy Soc B 360:1443–1455
Murphy CG, Gerhardt HC (2002) Mate sampling by female barking treefrogs (Hyla gratiosa). Behav Ecol 13:472–480
Neelon DP, Höbel G (2017) Social plasticity in choosiness in green tree frogs, Hyla cinerea. Behav Ecol (published online), https://doi.org/10.1093/beheco/arx103)
Prugh LR, Golden CD (2014) Does moonlight increase predation risk? Meta-analysis reveals divergent responses of nocturnal mammals to lunar cycles. J Anim Ecol 83:504–514
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Rand AS, Bridarolli ME, Dries L, Ryan MJ (1997) Light levels influence female choice in túngara frogs: predation risk assessment? Copeia 1997:447–450
Reichert MS, Höbel G (2015) Modality interactions alter the shape of acoustic mate preference functions in gray treefrogs. Evolution 69:2384–2398
Reichert MS, Symes LB, Höbel G (2016) Lighting up sound preferences: cross-modal influences on the precedence effect in treefrogs. Anim Behav 119:151–159
Ritchie MG (1992) Setbacks in the search for mate-preference genes. Trends Ecol Evol 7:328–329
Rodríguez RL, Ramaswamy K, Cocroft RB (2006) Evidence that female preferences have shaped male signal evolution in a clade of specialized plant-feeding insects. Proc R Soc Lond B 273:2585–2593
Rundus AS, Santer RD, Hebets EA (2010) Multimodal courtship efficacy of Schizocosa retrorsa wolf spiders: implications of an additional signal modality. Behav Ecol 21:701–707
Runkle LS, Wells KD, Robb CC, Lance SL (1994) Individual, nightly, and seasonal variation in calling behavior of the gray tree frog, Hyla versicolor: implications for energy expenditure. Behav Ecol 5:318–325
Ryan MJ (1985) The túngara frog: a study in sexual selection and communication. University of Chicago Press, Chicago
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schwartz JJ, Bee MA, Tanner SD (2000) A behavioral and neurobiological study of the responses of gray treefrogs, Hyla versicolor to the calls of a predator, Rana catesbeiana. Herpetologica 2000:27–37
Schwartz JJ, Buchanan BW, Gerhardt HC (2001) Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol 49:443–455
Sueur J, Aubin T, Simonis C (2008) Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics 18:213–226
Sullivan BK, Hinshaw SH (1992) Female choice and selection on male calling behaviour in the grey treefrog Hyla versicolor. Anim Behav 44:733–744
Taylor RC, Klein BA, Ryan MJ (2011) Inter-signal interaction and uncertain information in anuran multimodal signals. Curr Zool 57:153–161
Uetz GW, Norton S (2007) Preference for male traits in female wolf spiders varies with the choice of available males, female age and reproductive state. Behav Ecol Sociobiol 61:631–641
Wells KD (2010) The ecology and behavior of amphibians. University of Chicago Press, Chicago
West-Eberhard MJ (1983) Sexual selection, social competition, and speciation. Q Rev Biol 58:155–183
Acknowledgements
We thank D. Neelon, R. Gremminger, A. Heesacker, C. Robertson, and T. Trinklein for assistance in the field and in running playback trials. Staff at the University of Wisconsin-Milwaukee Field Station gave logistical support, and Nancy and Michael Byers graciously allowed us to collect frogs on their land. We also thank the reviewers for their time and suggestions on this manuscript.
Funding
The study was indirectly supported by the Office of Undergraduate Research of the University of Wisconsin–Milwaukee, by providing SURF fellowships to a number of undergraduate students.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All applicable national and institutional guidelines for the care and use of animals were followed. We received ethical clearance from the Institutional Animal Care and Use Committee of the University of Wisconsin–Milwaukee (protocol number 15–16 #43).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Additional information
Communicated by K. Summers
Electronic supplementary material
ESM 1
(XLSX 64 kb)
Rights and permissions
About this article
Cite this article
Underhill, V.A., Höbel, G. Variation in nocturnal light levels does not alter mate choice behavior in female eastern gray treefrogs (Hyla versicolor). Behav Ecol Sociobiol 71, 151 (2017). https://doi.org/10.1007/s00265-017-2386-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2386-1