Abstract
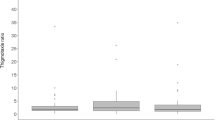
Cognitive abilities evolve by natural selection to help an organism cope with problems encountered in the organism’s typical environment. In acacia ants, coevolution with the acacia tree led workers to forage exclusively on the host plant (“in-nest” foraging), instead of the central-place foraging typical for most social insects. To test whether foraging ecology altered the orientation skills of acacia ants, we developed a novel field disorientation assay to evaluate the ability of foraging workers to quickly reorient after being disoriented (rotated) in an experimental arena. We compared 10 behaviors among disoriented and sham-treated workers of three in-nest foraging species (Pseudomyrmex nigrocinctus, P. flavicornis, and P. spinicola) and two central-place foraging species that regularly forage off the host tree (P. gracilis, P. nigropilosus). We predicted that experimental disorientation of workers should affect in-nest foraging species (acacia ants) more than central-place foraging species. Behavioral differences between control and disoriented ants were not consistently associated with foraging ecology, although the species least able to recover after disorientation was an acacia ant (P. nigrocinctus), and the species performing best after disorientation was a central-place forager (P. gracilis). Only one of the 10 behaviors studied consistently differed in experimentally disoriented workers compared to controls in all three species of acacia ants, whereas none of the experimentally disoriented central-place foragers differed from control workers for this specific behavior. Future studies could evaluate additional ant species living in obligate associations with plants, to further compare the cognitive abilities of in-nest versus central-place foraging organisms.
Significance statement
Foraging ecology influences the evolution of spatial orientation abilities. Acacia ants exclusively nest and forage on acacia trees; unlike most other ants that are central-place foragers, acacia ants therefore do not face the challenge of finding the way back home after collecting food. We compared the performance of three species of acacia ants to the performance of two species of central-place foragers on a disorientation assay in the field. We found that the ability to reorient was not consistently associated with foraging ecology, although the species least able to recover after disorientation was an acacia ant (P. nigrocinctus), and the species performing best after disorientation was a central-place forager (P. gracilis). Other behaviors related with the mutualism with the acacia tree—such as pruning nearby vegetation and falling off branches to attack potential herbivores—could also select for orientation abilities because workers reorient back to the host tree.





Similar content being viewed by others
References
Agostinelli C, Lund U (2013) R package “circular”: Circular Statistics (version 0.4-7). URL https://r-forge.r-project.org/projects/circular/
Amador-Vargas S (2012a) Plant killing by mutualistic ants increases the density of host species seedlings in the dry forest of Costa Rica. Psyche (Stuttg) 2012:28–33
Amador-Vargas S (2012b) Run, robber, run: parasitic acacia ants use speed and evasion to steal food from ant-defended trees. Physiol Entomol 37:323–329
Amador-Vargas S, Gronenberg W, Wcislo WT, Mueller U (2015) Specialization and group size: brain and behavioural correlates of colony size in ants lacking morphological castes. Proc R Soc Lond B Biol Sci 282:20142502. doi:10.1098/rspb.2014.2502
Balda RP, Kamil A (2006) Linking life zones, life history traits, ecology, and spatial cognition in four allopatric southwestern seed caching corvids. Pap Behav Biol Sci 36
Balda RP, Kamil AC (1989) A comparative study of cache recovery by three corvid species. Anim Behav 38:486–495
Balda RP, Kamil AC (2002) Spatial and social cognition in corvids: an evolutionary approach. Cogn Anim Empir Theor Perspect Anim Cogn 129–134
Bednekoff PA, Balda RP, Kamil AC, Hile AG (1997) Long-term spatial memory in four seed-caching corvid species. Anim Behav 53:335–341
Beekman M, Ratnieks FLW (2000) Long-range foraging by the honey-bee, Apis mellifera L. Funct Ecol 14:490–496
Blomberg SP, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Blumstein D, Evans C, Daniels J (2006) JWatcher 1.0. http://www.jwatcher.ucla.edu/
Bolhuis JJ, Macphail EM (2001) A critique of the neuroecology of learning and memory. Trends Cogn Sci 5:426–433
Brown D (2009) Tracker Video Analysis and Modeling Tool (Version 4.92)
Bühlmann C, Cheng K, Wehner R (2011) Vector-based and landmark-guided navigation in desert ants inhabiting landmark-free and landmark-rich environments. J Exp Biol 214:2845–2853. doi:10.1242/jeb.054601
Cheng K, Schultheiss P, Schwarz S et al (2014) Beginnings of a synthetic approach to desert ant navigation. Behav Process 102:51–61
Chomicki G, Ward PS, Renner SS (2015) Macroevolutionary assembly of ant-plant symbioses: Pseudomyrmex ants and their ant-housing plants in the Neotropics. Proc R Soc B 282:2015–2200
Clayton NS, Krebs JR (1994) Memory for spatial and object-specific cues in food-storing and non-storing birds. J Comp Physiol A 174:371–379. doi:10.1007/BF00240218
Clement LW, Köppen SCW, Brand WA, Heil M (2008) Strategies of a parasite of the ant–Acacia mutualism. Behav Ecol Sociobiol 62:953–962. doi:10.1007/s00265-007-0520-1
Collett M, Chittka L, Collett TS (2013) Spatial memory in insect navigation. Curr Biol 23:R789–R800
Cristol DA, Reynolds EB, Leclerc JE et al (2003) Migratory dark-eyed juncos, Junco hyemalis, have better spatial memory and denser hippocampal neurons than nonmigratory conspecifics. Anim Behav 66:317–328
Day L, Crews D, Wilczynski W (1999) Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim Behav 57:393–407
Davidson DW, Longino JT, Snelling RR (1988) Pruning of host plant neighbors by ants: an experimental approach. Ecology 69:801–808
Dyer FC (1998) Spatial cognition: lessons from central-place foraging insects. In: Animal cognition in nature: The Convergence of Psychology and Biology in Laboratory and Field, R. P. Balda, I. M. Pepperberg & A. C. Kamil. pp 119–154
Frederickson ME, Greene MJ, Gordon DM (2005) “Devil”s gardens’ bedevilled by ants. Nature 437:495–496
Fritz SA, Purvis A (2010) Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv Biol 24:1042–1051
Gómez-Acevedo S, Rico-Arce L, Delgado-Salinas A et al (2010) Neotropical mutualism between Acacia and Pseudomyrmex: phylogeny and divergence times. Mol Phylogenet Evol 56:393–408
Hanson PE, Longino JT (2006) Hormigas. In: Hymenoptera de la región neotropical, Hanson, P. & Gauld, I. American Entomological Institute, p 994
Hilton SC, Krebs JK (1990) Spatial memory of four species of Parus: performance in an open-field analogue of a radial maze. Q J Exp Psychol Sect B 42:345–368
Huber R, Knaden M (2015) Egocentric and geocentric navigation during extremely long foraging paths of desert ants. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 201:609–616. doi:10.1007/s00359-015-0998-3
Izzo T, Vasconcelos H (2002) Cheating the cheater: domatia loss minimizes the effects of ant castration in an Amazonian ant-plant. Oecologia 133:200–205
Janzen DH (1966) Coevolution of mutualism between ants and acacias in Central America. Evolution 20:249–275
Janzen DH (1974) Swollen-thorn acacias of Central America. Smithson Contrib Bot 13:1–131
Janzen DH (1975) Pseudomyrmex nigropilosa: a parasite of a mutualism. Science 188:936–937
Knaden M, Graham P (2016) The sensory ecology of ant navigation: from natural environments to neural mechanisms. Annu Rev Entomol 61:63–76. doi:10.1146/annurev-ento-010715-023703
Lefebvre L (1995) Ecological correlates of social learning: problems and solutions for the comparative method. Behav Process 35:163–171
Macphail EM (1982) Brain and intelligence in vertebrates. Clarendon Press, Oxford
Morawetz W, Henzl M, Wallnofer B (1992) Tree killing by herbicide producing ants for the establishment of pure Tococa occidentalis populations in the Peruvian Amazon. Biodivers Conserv 1:19–33
Odling-Smee LC, Boughman JW, Braithwaite VA (2008) Sympatric species of threespine stickleback differ in their performance in a spatial learning task. Behav Ecol Sociobiol 62:1935–1945
Orians GH, Pearson NE (1979) On the theory of central place foraging. Anal Ecol Syst 155–177
Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W (2013) caper: Comparative Analyses of Phylogenetics and Evolution in R. R package version 0.5.2. http://CRAN.R-project.org/package=caper
Pahl M, Zhu H, Tautz J, Zhang S (2011) Large scale homing in honeybees. PLoS One 6:e19669. doi:10.1371/journal.pone.0019669
Renner SS, Ricklefs RE (1998) Herbicidal activity of domatia-inhabiting ants in patches of Tococa guianensis and Clidemia heterophylla. Biotropica 30:324–327
Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Rico-Gray V, Oliveira PS (2007) The ecology and evolution of ant-plant interactions. University of Chicago Press, USA
Rosati AG, Rodriguez K, Hare B (2014) The ecology of spatial memory in four lemur species. Anim Cogn 17:947–961
Schultheiss P, Schwarz S, Cheng K, Wehner R (2013) Foraging ecology of an Australian salt-pan desert ant (genus Melophorus). Aust J Zool 60:311–319
Schwarz S, Cheng K (2010) Visual associative learning in two desert ant species. Behav Ecol Sociobiol 64:2033–2041. doi:10.1007/s00265-010-1016-y
Sherry DF (2006) Neuroecology. Annu Rev Psychol 57:167–197
Shettleworth SJ (2009) Cognition, evolution, and behavior. Oxford University Press, USA
Shettleworth SJ (1990) Spatial memory in food-storing birds. Philos Trans R Soc Lond Ser B Biol Sci 329:143–151
Smulders TV, Gould KL, Leaver LA (2010) Using ecology to guide the study of cognitive and neural mechanisms of different aspects of spatial memory in food-hoarding animals. Philos Trans R Soc Lond Ser B Biol Sci 365:883–900
Steck K, Hansson BS, Knaden M (2009) Smells like home: desert ants, Cataglyphis fortis, use olfactory landmarks to pinpoint the nest. Front Zool 6:5
Steck K, Hansson BS, Knaden M (2011) Desert ants benefit from combining visual and olfactory landmarks. J Exp Biol 214:1307–1312. doi:10.1242/jeb.053579
Ward PS (2014) The phylogeny and evolution of ants. Annu Rev Ecol Evol Syst 45:23–43
Wehner R (2009) The architecture of the desert ant’s navigational toolkit (Hymenoptera: Formicidae). Myrmecol News 12:85–96
Wystrach A, Beugnon G, Cheng K (2012) Ants might use different view-matching strategies on and off the route. J Exp Biol 215:44–55
Wystrach A, Schwarz S, Baniel A, Cheng K (2013) Backtracking behaviour in lost ants: an additional strategy in their navigational toolkit. Proc R Soc B Biol Sci 280:20131677. doi:10.1098/rspb.2013.1677
Wystrach A, Philippides A, Aurejac A et al (2014) Visual scanning behaviours and their role in the navigation of the Australian desert ant Melophorus bagoti. J Comp Physiol A 200:615–626. doi:10.1007/s00359-014-0900-8
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, USA
Zeil J, Narendra A, Stürzl W (2014) Looking and homing: how displaced ants decide where to go. Philos Trans R Soc B Biol Sci 369:20130034. doi:10.1098/rstb.2013.0034
Zurbuchen A, Landert L, Klaiber J et al (2010) Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv 143:669–676
Acknowledgments
We thank Natalia Ramírez Amador and Marianela Solís del Valle for field assistance; William Eberhard and Gilbert Barrantes for discussion of ideas; the staff at OTS Palo Verde Research Station, park rangers and administration of Palo Verde National Park for their support; Rodolfo Amador for his help in designing the experimental disc; and Will Shim, Chad Smith, and two anonymous reviewers for comments that greatly improved the manuscript. An EEB fellowship of the University of Texas at Austin, the Christiane and Christopher Tyson Fellowship awarded by the Organization for Tropical Studies, and NSF-DDIG award 1210412 to SAV financed this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Research was conducted under scientific permits 08-2012-SINAC and SE-PI-R-139-2013 from MINAET in accordance with the laws of the Republic of Costa Rica.
Additional information
Communicated by W. Hughes
Electronic supplementary material
ESM 1
(DOCX 479 kb)
Rights and permissions
About this article
Cite this article
Amador-Vargas, S., Mueller, U.G. Ability to reorient is weakly correlated with central-place versus non-central-place foraging in acacia ants. Behav Ecol Sociobiol 71, 43 (2017). https://doi.org/10.1007/s00265-016-2262-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-016-2262-4