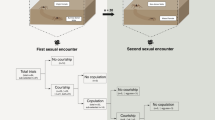
Abstract
Courting, accessing, and/or competing for mates are involved in sexual selection by generating differences in mating success. Although courtship behavior should reflect intensity of mating competition and sexual selection, studies that compare courtship behavior across populations/species with different mating systems subject to differing degrees of mating competition are scanty. Here, we compare courtship behavior between two closely related plover species (Charadrius spp.): a polygamous population of snowy plovers and a socially monogamous population of Kentish plovers. Consistently with expectations, both males and females spent more time courting in the polygamous plover than in the monogamous one. In addition, courtship behavior of males relative to females increased over the breeding season in the polygamous plover, whereas it did not change in the monogamous one. Our results therefore suggest that courtship behavior is a fine-tuned and informative indicator of sexual selection in nature.


Similar content being viewed by others
References
Adkins-Regan E, Tomaszycki M (2007) Monogamy on the fast track. Biol Lett 3:617–619
AlRashidi M, Kosztolányi A, Shobrak M, Székely T (2011) Breeding ecology of the Kentish plover, Charadrius alexandrinus, in the Farasan Islands, Saudi Arabia. Zool Middle East 53:15–24
Amat JA, Fraga RM, Arroyo GM (1999) Brood desertion and polygamous breeding in the Kentish plover, Charadrius alexandrinus. Ibis 141:596–607
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, NJ
Baker RH, Wilkinson GS (2001) Phylogenetic analysis of sexual dimorphism and eye-span allometry in stalk-eyed flies (Diopsidae). Evolution 55:1373–1385
Birkhead TR, Atkin L, Møller AP (1987) Copulation behaviour of birds. Behaviour 101:101–138
Clark CJ (2012) The role of power versus energy in courtship: what is the ‘energetic cost’ of a courtship display? Anim Behav 84:269–277
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123:212–229
Cramp S, Simmons KEL (1985) Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic, vol III. Oxford University Press, Oxford, Waders to gulls
Crawley MJ (2003) Statistical computing: an introduction to data analysis using S-plus. Wiley, Chichester
Dobson FS, Nolan PM, Nicolaus M, Bajzak C, Coquel A-S, Jouventin P (2008) Comparison of color and body condition between early and late breeding king penguins. Ethology 114:925–933
Dos Remedios N, Lee PLM, Burke T, Székely T, Küpper C (2015) North or south? Phylogenetic and biogeographic origins of a globally distributed avian clade. Mol Phylogenet Evol 89:151–159
Dunn PO, Whittingham LA, Pitcher TE (2001) Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution 55:161–175
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Fairbairn D, Blanckenhorn W, Székely T (2007) Sex, size and gender roles. Evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford
Forsgren E, Amundsen T, Borg AA, Bjelvenmark J (2004) Unusually dynamic sex roles in a fish. Nature 429:551–554
Fritzsche K, Arnqvist G (2013) Homage to Bateman: sex roles predict sex differences in sexual selection. Evolution 67:1926–1936
Grant PR, Grant BR (2002) Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296:707–711
Hankison SJ, Ptacek MB (2007) Within and between species variation in male mating behaviors in the Mexican sailfin mollies Poecilia velifera and P. petenensis. Ethology 113:802–812
Hollis B, Kawecki TJ (2014) Male cognitive performance declines in the absence of sexual selection. Proc R Soc B 281:20132873
Jennions M, Kokko H (2010) Sexual selection. In: Westeneat D, Fox C (eds) Evolutionary behavioral ecology. Oxford University Press, New York, pp 343–364
Kasumovic MM, Bruce MJ, Andrade MCB, Herberstein ME (2008) Spatial and temporal demographic variation drives within-season fluctuations in sexual selection. Evolution 62:2316–2325
Kasuya E (2001) Mann–Whitney U test when variances are unequal. Anim Behav 61:1247–1249
Kokko H, Klug H, Jennions MD (2012) Unifying cornerstones of sexual selection: operational sex ratio, Bateman gradient and the scope for competitive investment. Ecol Lett 15:1340–1351
Kosztolányi A, Javed S, Küpper C, Cuthill IC, Al Shamsi A, Székely T (2009) Breeding ecology of Kentish plover Charadrius alexandrinus in an extremely hot environment. Bird Study 56:244–252
Kotiaho JS (2002) Sexual selection and condition dependence of courtship display in three species of horned dung beetles. Behav Ecol 13:791–799
Küpper C, Augustin J, Kosztolányi A, Burke T, Figuerola J, Székely T (2009) Kentish versus snowy plover: phenotypic and genetic analyses of Charadrius alexandrinus reveal divergence of Eurasian and American subspecies. Auk 126:839–852
Kvarnemo C, Ahnesjö I (1996) The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408
Lessells CM (1984) The mating system of Kentish plovers Charadrius alexandrinus. Ibis 126:474–483
Liker A, Freckleton RP, Szekely T (2013) The evolution of sex roles in birds is related to adult sex ratio. Nat Commun 4:1587
Liker A, Freckleton RP, Székely T (2014) Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr Biol 24:880–884
Lindenfors P, Tullberg BS (1998) Phylogenetic analyses of primate size evolution: the consequences of sexual selection. Biol J Linn Soc 64:413–447
Martin P, Bateson P (2009) Measuring behaviour an introductory guide. Cambridge University Press, Cambridge
Møller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments? Behav Ecol Sociobiol 32:167–176
Myhre LC, Kd J, Forsgren E, Amundsen T (2012) Sex roles and mutual mate choice matter during mate sampling. Am Nat 179:741–755
O’Brien E, Dawson R (2013) Experimental dissociation of individual quality, food and timing of breeding effects on double-brooding in a migratory songbird. Oecologia 172:689–699
Ord TJ, Blumstein DT, Evans CS (2001) Intrasexual selection predicts the evolution of signal complexity in lizards. Proc R Soc Lond B 268:737–744
Page GW, Stenzel LE, Warriner JS, Warriner JC, Paton PW (2009) Snowy plover (Charadrius nivosus). In: Poole A (ed) The Birds of North America Online. Cornell Lab of Ornithology, Ithaca, http://bna.birds.cornell.edu/bna/species/154/articles/introduction
Pariser EC, Mariette MM, Griffith SC (2010) Artificial ornaments manipulate intrinsic male quality in wild-caught zebra finches (Taeniopygia guttata). Behav Ecol 21:264–269
Parra JE, Beltrán M, Zefania S, Dos Remedios N, Székely T (2014) Experimental assessment of mating opportunities in three shorebird species. Anim Behav 90:83–90
Pedroso SS, Barber I, Svensson O, Fonseca PJ, Amorim MCP (2013) Courtship sounds advertise species identity and male quality in sympatric Pomatoschistus spp. gobies. PLoS One 8:e64620
Pérez-Barbería FJ, Gordon IJ, Pagel M (2002) The origins of sexual dimorphism in body size in ungulates. Evolution 56:1276–1285
Price JJ, Whalen LM (2009) Plumage evolution in the oropendolas and caciques: different divergence rates in polygynous and monogamous taxa. Evolution 63:2985–2998
Quinn VS, Hews DK (2010) The evolutionary decoupling of behavioral and color cues in a multicomponent signal in two Sceloporus lizards. Ethology 116:509–516
Reynolds JD (1993) Should attractive individuals court more? Theory and a test. Am Nat 141:914–927
Rosenqvist G, Berglund A (2011) Sexual signals and mating patterns in Syngnathidae. J Fish Biol 78:1647–1661
Sánchez-Macouzet O, Rodríguez C, Drummond H (2014) Better stay together: pair bond duration increases individual fitness independent of age-related variation. Proc R Soc B 281:20132843
Shuster SM (2009) Sexual selection and mating systems. Proc Natl Acad Sci USA 106:10009–10016
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Siepielski AM, DiBattista JD, Carlson SM (2009) It’s about time: the temporal dynamics of phenotypic selection in the wild. Ecol Lett 12:1261–1276
Stenzel LE, Page GW, Warriner JC, Warriner JS, Neuman KK, George DE, Eyster CR, Bidstrup FC (2011) Male-skewed adult sex ratio, survival, mating opportunity and annual productivity in the snowy plover Charadrius alexandrinus. Ibis 153:312–322
Székely T, Cuthill IC, Kis J (1999) Brood desertion in Kentish plover: sex differences in remating opportunities. Behav Ecol 10:185–190
Székely T, Kosztolányi A, Küpper C (2008) Practical guide for investigating breeding ecology of Kentish plover Charadrius alexandrinus. Unpublished Report, University of Bath, Bath, http://www.bath.ac.uk/bio-sci/biodiversity-lab/pdfs/KP_Field_Guide_v3.pdf
Székely T, Reynolds JD, Figuerola J (2000) Sexual size dimorphism in shorebirds, gulls, and alcids: the influence of sexual and natural selection. Evolution 54:1404–1413
Thorén S, Lindenfors P, Kappeler PM (2006) Phylogenetic analyses of dimorphism in primates: evidence for stronger selection on canine size than on body size. Am J Phys Anthropol 130:50–59
van den Assem J, Werren JH (1994) A comparison of the courtship and mating behavior of three species of Nasonia (Hymenoptera: Pteromalidae). J Insect Behav 7:53–66
Verhulst S, Nilsson J-Å (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos T Roy Soc B 363:399–410
Vincze O, Székely T, Küpper C et al (2013) Local environment but not genetic differentiation influences biparental care in ten plover populations. PLoS One 8:e60998
Wachtmeister C-A (2001) Display in monogamous pairs: a review of empirical data and evolutionary explanations. Anim Behav 61:861–868
Wang S, Cummings M, Kirkpatrick M (2015) Coevolution of male courtship and sexual conflict characters in mosquito fish. Behav Ecol (published online, doi:10.1093/beheco/arv049)
Warriner JS, Warriner JC, Page GW, Stenzel LE (1986) Mating system and reproductive success of a small population of polygamous snowy plovers. Wilson Bull 98:15–37
Weir LK, Grant JWA, Hutchings JA (2011) The influence of operational sex ratio on the intensity of competition for mates. Am Nat 177:167–176
Wilson AB, Ahnesjö I, Vincent ACJ, Meyer A (2003) The dynamics of male brooding, mating patterns, and sex roles in pipefishes and seahorses (family Syngnathidae). Evolution 57:1374–1386
Acknowledgments
We thank all fieldwork volunteers and people that have worked and supported the conservation project of snowy plovers at Bahía de Ceuta, especially to M. Cruz-Lopéz, I. Guardado, and O. Castañeda. We also thank the Universidad Autónoma de Sinaloa for logistic support through the program PROTORMAR. The snowy plover project fieldwork permit for Ceuta was provided by SEMARNAT (SGPA/DGVS/03076/13). We thank also the numerous volunteers who collected data for the Kentish plover project in Maio, Cape Verde, especially A. Tavares and E. Inés; we also thank the support of all the Fundação Maio Biodiversidade (FMB) team, DGA (who provided permit to work on Maio) and the Camara Municipal do Maio. Funding for fieldwork in Ceuta was given to MAS-M by CONACyT (Consejo Nacional de Ciencia y Tecnología, Mexico, Ciencia Básica 2010, número de proyecto 157570); funding for fieldwork in Maio was provided by a DFG Mercator Visiting Professorship awarded to TS. CK was supported by a Marie Curie intraeuropean postdoctoral fellowship. MAS-M was supported by the Posgrado en Ciencias Biológicas of the Universidad Autónoma de Tlaxcala. This study is part of the PhD dissertation of MCC-I, who was funded by CONACyT (scholarship number 216052/311485) and is grateful to H. Drummond for lending field equipment and to S. Ancona and N. dos Remedios for their useful comments on earlier versions of this manuscript. We thank the thorough comments of two anonymous reviewers which improved our manuscript substantially.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
In this study, we investigated two geographically widespread and non-endangered bird species in their natural habitats. The data collected for this study were based only on observations that did not require any capture or manipulation; data collection consisted in observations of pairs at a distance of 10 to 20 m using a hide or a car, carefully avoiding the disturbance of the normal activities of birds. When using a car, we drove only on marked tracks where cars usually drive in order to avoid additional disturbance of the natural habitat. As part of the annual monitoring, birds were caught using funnel traps during late incubation or after hatching of the clutch. Traps were left on the nest/clutch up to 25 min and were shaded to avoid egg exposure to heat; trapping was avoided at extreme heat. Birds were ringed and manipulated by well-trained people. All aspects of the fieldwork were authorized by the national authorities in Cape Verde (Direcção Geral do Ambiente, DGA) and Mexico (Secretaría de Medio Ambiente y Recursos Naturales, SEMARNAT).
Additional information
Communicated by C. M. Garcia
Rights and permissions
About this article
Cite this article
Carmona-Isunza, M.C., Küpper, C., Serrano-Meneses, M.A. et al. Courtship behavior differs between monogamous and polygamous plovers. Behav Ecol Sociobiol 69, 2035–2042 (2015). https://doi.org/10.1007/s00265-015-2014-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-2014-x