Abstract
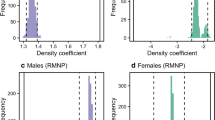
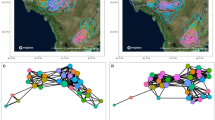
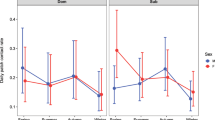
Although pathogen transmission dynamics are profoundly affected by population social and spatial structure, few studies have empirically demonstrated the population-level implications of such structure in wildlife. In particular, epidemiological models predict that the extent to which contact patterns are clustered decreases a pathogen’s ability to spread throughout an entire population, but this effect has yet to be demonstrated in a natural population. Here, we use network analysis to examine patterns of transmission of an environmentally transmitted parasite, Cryptosporidium spp., in Belding’s ground squirrels (Spermophilus beldingi). We found that the prevalence of Cryptosporidium was negatively correlated with transitivity, a measure of network clustering, and positively correlated with the percentage of juvenile males. Additionally, network transitivity decreased when there were higher percentages of juvenile males; the exploratory behavior demonstrated by juvenile males may have altered the structure of the network by reducing clustering, and low clustering was associated with high prevalence. We suggest that juvenile males are critical in mediating the ability of Cryptosporidium to spread through colonies, and thus may function as “super-spreaders.” Our results demonstrate the utility of a network approach in quantifying mechanistically how differences in contact patterns may lead to system-level differences in infection patterns.




Similar content being viewed by others
References
Ames GM, George DB, Hampson CP, Kanerek AR, McBee CD, Lockwood DR, Achter JD, Webb CT (2011) Using network properties to predict disease dynamics on human contact networks. Proc R Soc Lond B 278:3544–2550
Anderson RM, May RM (1992) Infectious diseases of humans. Oxford University, Oxford
Atwill ER, Camargo SM, Phillips R, Alonso LH, Tate KW, Jensen WA, Bennet J, Little S, Salmon TP (2001) Quantitative shedding of two genotypes of Cryptosporidium parvum in California ground squirrels (Spermophilus beecheyi). Appl Environ Microbiol 67:2840–2843
Atwill ER, Phillips R, Pereira MGC, Li X, McCowan B (2004) Seasonal shedding of multiple Cryptosporidium genotypes in California ground squirrels (Spermophilus beecheyi). Appl Environ Microbiol 70:6748–6752
Badham J, Stocker R (2010) The impact of network clustering and assortivity on epidemic behaviour. Theor Popul Biol 77:71–75
Bansal S, Grenfell BT, Meyers LA (2007) When individual behaviour matters: homogeneous and network models in epidemiology. J R Soc Interface 4:879–891
Benarska M, Bajer A, Kulis K, Sinski E (2003) Biological characterisation of Cryptosporidium parvum isolates of wildlife rodents in Poland. Ann Agric Environ Med 10:163–169
Burnham KP, Anderson DR (2002) Model selection and multimodal inference: a practical information-theoretic approach. Springer, New York
Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JMJ (1997) Social ties and susceptibility to the common cold. JAMA-J Am Med Assoc 277:1940–1944
Corner LAL, Pfeiffer DU, Morris RS (2003) Social-network analysis of Mycobacterium bovis transmission among captive brushtail possums (Trichosurus vulpecula). Prev Vet Med 59:147–167
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton University, Princeton
Croft DP, Madden JR, Franks DW, James R (2011) Hypothesis testing in animal social networks. Trends Ecol Evol 26:502–507
Drewe JA (2009) Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Proc R Soc Lond B 277:633–642
Fenner AL, Godfrey SS, Bull CM (2011) Using social networks to deduce whether residents or dispersers spread parasites in a lizard population. J Anim Ecol 80:835–843
Ferrari N, Cattadori IM, Nespereira J, Rizzoli A, Hudson PJ (2003) The role of host sex in parasite dynamics: field experiments on the yellow-necked mouse Apodemus flavicollis. Ecol Lett 6:1–7
Godfrey SS, Bull CM, James R, Murray K (2009) Network structure and parasite transmission in a group living lizard, gidgee skink, Egernia stokesii. Behav Ecol Sociobiol 63:1045–1056
Godfrey SS, Moore JA, Nelson NJ, Bull CM (2010) Social network structure and parasite infection patterns in a territorial reptile, the tuatara (Sphenodon punctatus). Int J Parasitol 40:1575–1585
Grear DA, Perkins SE, Hudson PJ (2009) Does elevated testosterone result in increased exposure and transmission of parasites? Ecol Lett 12:528–537
Hawley DM, Etienne RS, Ezenwa VO, Jolles AE (2011) Does animal behavior underlie covariation between hosts’ exposure to infectious agents and susceptibility to infection? Implications for disease dynamics. Integr Comp Biol 5:528–539
Holekamp K (1984) Dispersal in ground-dwelling Sciurids. In: Murie JO, Michener GR (eds) The Biology of Ground-dwelling Squirrels. University of Nebraska, Lincoln, pp 295–320
Hou L, Li X, Moeller R, Palermo B, Atwill ER (2004) Neonatal-mouse infectivity of intact Cryptosporidium parvum oocysts isolated after optimized in vitro excystation. Appl Environ Microbiol 70:642–646
Keeling MJ (1999) The effects of local spatial structure on epidemiological invasions. Proc R Soc Lond B 266:859–867
Keeling MJ (2005) The implications of network structure for epidemic dynamics. Theor Popul Biol 67:1–8
Keeling MJ, Eames KTD (2005) Networks and epidemic models. J R Soc Interface 2:295–307
Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM (2005) Superspreading and the effect of individual variation on disease emergence. Nature 438:355–359
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled? Trends Ecol Evol 16:295–300
McLean IG (1984) Spacing behavior and aggression in female ground squirrels. In: Murie JO, Michener GR (eds) The biology of ground-dwelling squirrels. University of Nebraska, Lincoln, pp 321–335
Michener GR (1984) Age, sex, and species differences in the annual cycles of ground-dwelling Sciurids: implications for sociality. In: Murie JO, Michener GR (eds) The biology of ground-dwelling squirrels. University of Nebraska, Lincoln, pp 79–107
Murie JO, Michener GR (1984) Biology of ground-dwelling squirrels. University of Nebraska, Lincoln
Newman MEJ (2003) Properties of highly clustered networks. Phys Rev E 68:1–6
Nunes S, Muecke EM, Anthony JA, Batterbee AS (1999) Endocrine and energetic mediation of play behavior in free-living Belding’s ground squirrels. Horm Behav 36:153–165
Otterstatter MC, Thomson JD (2007) Contact networks and transmission of an intestinal pathogen in bumble bee (Bombus impatiens) colonies. Oecologia 154:411–421
Perkins SE, Ferrari MF, Hudson PJ (2008) The effects of social structure and sex-biased transmission on macroparasite infection. Parasitology 135:1561–1569
Perkins SE, Cagnacci F, Stradiotto A, Arnoldi D, Hudson PJ (2009) Comparison of social networks derived from ecological data: implications for inferring infectious disease dynamics. J Anim Ecol 78:1015–1022
Porphyre T, McKenzie J, Stevenson MA (2011) Contact patterns as a risk factor for bovine tuberculosis infection in a free-living adult brushtail possum Trichosurus vulpecula population. Prev Vet Med 100:221–230
Turner J, Bowers JA, Clancy D, Behnke MC, Christley RM (2008) A network model of E coli O157 transmission within a typical UK dairy herd: the effect of heterogeneity and clustering on the prevalence of infection. J Theor Biol 254:45–54
Valente TW (2010) Social networks and health: models, methods, and applications. Oxford University, Oxford
Wasserman S, Faust K (1994) Social network analysis: methods and applications. Cambridge University, Cambridge
Wu X, Liu Z (2008) How community structure influences epidemic spread in social networks. Physica A 387:623–630
Zu S-X, Fang G-D, Fayer R, Guerrant RL (1992) Cryptosporidiosis: pathogenesis and immunology. Parasitol Today 8:24–27
Acknowledgments
We thank Jennifer Dike and Katryna Fleer for their assistance in data collection and Allison Heagerty for her comments and contributions to data analysis. We also thank two anonymous reviewers for their constructive comments on an earlier version of this manuscript. This work was conducted under the auspices of the Bernice Barbour Communicable Disease Laboratory, with financial support from the Bernice Barbour Foundation, Hackensack, N.J., as a grant to the Center of Equine Health, University of California, Davis.
Ethical standards
The experiments described herein comply with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. P. Croft
Rights and permissions
About this article
Cite this article
VanderWaal, K.L., Atwill, E.R., Hooper, S. et al. Network structure and prevalence of Cryptosporidium in Belding’s ground squirrels. Behav Ecol Sociobiol 67, 1951–1959 (2013). https://doi.org/10.1007/s00265-013-1602-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1602-x