Abstract
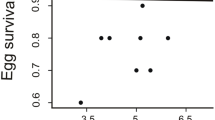
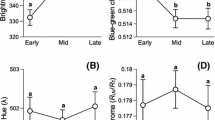
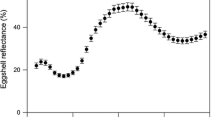
Carotenoid-based ornaments (many yellow–orange–red colourations) may signal the genetic or parental quality of the bearer. Thus, their expression could influence the amount of resources/energy that the mate will invest in the production of offspring, thereby optimising its reproductive fitness. The differential allocation hypothesis (DAH) predicts that females mated with more attractive males should lay more and better eggs. This has been explored only in few bird species with carotenoid-based traits. We tested this hypothesis in the red-legged partridge (Alectoris rufa), a gallinacean with very variable laying capacity. Both sexes display carotenoid-based ornamentation that gradually fades throughout the laying period. Here, the redness of beak and eye rings of captive males was intensified after mating by means of paint. The proportion of females that laid eggs did not differ between treatments. Amongst laying females, those mated with colour-enhanced males (experimental females) tended to lay earlier and produced significantly more eggs than controls, but of similar quality (egg mass and composition). We additionally investigated whether male attractiveness influenced egg components depending on the clutch size and laying sequence. The testosterone level in eggs from experimental females was positively related to the laying order, whereas control eggs did not show any trend. Our results provided mixed support for the DAH, but nevertheless revealed that female red-legged partridges may adjust their breeding investment according to male carotenoid-based ornamentation.



Similar content being viewed by others
References
Alonso-Alvarez C (2001) Effects of testosterone implants on pair behaviour during incubation in the yellow-legged gull Larus cachinnans. J Avian Biol 32:326–332
Alonso-Alvarez C, Pérez-Rodríguez L, Mateo R, Chastel O, Viñuela J (2008) The oxidation handicap hypothesis and the carotenoid allocation trade-off. J Evol Biol 21:1789–1797
Alonso-Alvarez C, Pérez-Rodríguez L, García JT, Viñuela J (2009) Testosterone-mediated trade-offs in the old age: a new approach to the immunocompetence handicap and carotenoid-based sexual signalling. Proc R Soc Lond B 276:2093–2101
Andersson M (1994) Sexual selection. Monographs in behaviour and ecology. Princeton University Press, Princettablon, NJ
Ardia DR, Broughton DR, Gleicher MJ (2010) Short-term exposure to testosterone propionate leads to rapid bill color and dominance changes in zebra finches. Horm Behav 58:526–532
Bolund E, Schielzeth H, Forstmeier W (2009) Compensatory investment in zebra finches: females lay larger eggs when paired to sexually unattractive males. Proc R Soc Lond B 276:707–715
Bortolotti GR, Negro JJ, Surai PF, Prieto P (2003) Carotenoids in eggs and plasma of red-legged partridges: effects of diet and reproductive output. Physiol Biochem Zool 76:367–374
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445
Carere C, Balthazart J (2007) Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol Metab 18:73–80
Casas F (2008) Gestión agraria y cinegética: efectos sobre la perdiz roja (Alectoris rufa) y aves esteparias protegidas. PhD thesis, University of Castilla La Mancha, Ciudad Real, Spain
Casas F, Morrish D, Viñuela J (2006) Paternidad extra-pareja en perdiz roja (Alectoris rufa). In XI Congreso Nacional y VIII Iberoamericano de Etología, Tenerife, Spain
Casas F, Mougeot F, Viñuela J (2009) Double-nesting behaviour and sexual differences in breeding success in wild red-legged partridges Alectoris rufa. IBIS 151:743–751
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Cramp S, Simmons K (1980) Red-legged partridge. In: Cramp S, Simmons K (eds) Handbook of the birds of Europe, the Middle East and North of Africa. The birds of the western Paleartic. Oxford University Press, Oxford, pp 463–439
Cucco M, Guasco B, Malacarne G, Ottonelli R, Tanvez A (2008) Yolk testosterone levels and dietary carotenoids influence growth and immunity of grey partridge chicks. Gen Comp Endocrinol 156:418–425
Dentressangle F, Boeck L, Torres R (2008) Maternal investment in eggs is affected by male feet colour and breeding conditions in the blue-footed booby, Sula nebouxii. Behav Ecol Sociobiol 62:1899–1908
Edward DA, Chapman T (2011) Mechanisms underlying reproductive trade-offs: Costs of reproduction. In Flatt T, Heyland A (eds) Mechanisms of life history evolution. The genetics and physiology of life-history trade-offs. Oxford University Press, Oxford, pp. 137–152
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evolution 34:76–91
Forstmeier W, Schielzeth H (2011) Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55
Galeotti P, Rubolini D, Fea G, Ghia D, Nardi PA, Gherardi F, Fasola M (2006) Female freshwater crayfish adjust egg and clutch size in relation to multiple male traits. Proc R Soc Lond B 273:1105–1110
Gautier P, Barroca M, Bertrand S, Eraud C, Gaillard M, Hamman M, Motreuil S, Sorci G, Faivre B (2008) The presence of females modulates the expression of a carotenoid-based sexual signal. Behav Ecol Sociobiol 62:1159–1166
Gil D (2008) Hormones in avian eggs: physiology, ecology and behavior. Adv Study Behav 38:337–398
Gil D, Graves J, Hazon N, Wells A (1999) Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286:126–128
Gilbert L, Williamson KA, Hazon N, Graves JA (2006) Maternal effects due to male attractiveness affect offspring development in the zebra finch. Proc R Soc Lond B 273:1765–1771
Giraudeau M, Duval C, Czirják GA, Bretagnolle V, Eraud C, McGraw KJ, Heeb P (2011) Maternal investment of female mallards is influenced by male carotenoid-based coloration. Proc R Soc Lond B 278:781–8
Gowaty PA (2003) Power asymmetries between the sexes, mate preferences, and components of fitness. In: Travis C (ed) Women, evolution and rape. MIT Press, Cambridge, MA, pp 61–86
Gowaty PA (2008) Reproductive compensation. J Evol Biol 21:1189–1200
Gowaty PA, Anderson WW, Bluhm CK, Drickamer LC, Kim YK, Moore AJ (2007) The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc Natl Acad Sci USA 104:15023–15027
Green RE (1984) Double nesting of the red-legged partridge Alectoris rufa. IBIS 126:332–346
Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C (2005) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352
Harris WE, Uller T (2009) Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Philos Trans R Soc B 364:1039–1048
Hegyi G, Herenyi M, Szollosi E, Rosivall B, Torok J, Groothuis TGG (2011) Yolk androstenedione, but not testosterone, predicts offspring fate and reflects parental quality. Behav Ecol 22:29–38
Helfenstein F, Losdat S, Saladin V, Richner H (2008) Females of carotenoid-supplemented males are more faithful and produce higher quality offspring. Behav Ecol 19:1165–1172
Hill GE (1990) Female house finches prefer colourful males: sexual selection for a condition-dependent trait. Anim Behav 40:563–572
Hill GE (2006) Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration. Function and evolution. Harvard University Press, Cambridge, pp 137–200
Hinde CA, Kilner RM (2007) Negotiations within the family over the supply of parental care. Proc R Soc Lond B 274:53–60
Horvathova T, Nakagawa S, Uller T (2012) Strategic female reproductive investment in response to male attractiveness in birds. Proc R Soc Lond B 279:163–170
Houde AE (1997) Sex, color, and mate choice in guppies. Princeton University Press, New Jersey
Houston AI, Székely T, McNamara JM (2005) Conflict between parents over care. Trends Ecol Evol 20:33–38
Ketterson EL, Nolan V (1999) Adaptation, exaptation and constraint: a hormonal perspective. Am Nat 154:S4–S25
Kingma SA, Komdeur J, Vedder O, von Engelhardt N, Korsten P, Groothuis TGG (2009) Manipulation of male attractiveness induces rapid changes in avian maternal yolk androgen deposition. Behav Ecol 20:172–179
Kotiaho JS, Simmons LW, Hunt J, Tomkins JL (2003) Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am Nat 161:852–859
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006). SAS for mixed models, second edition. SAS Institute Inc, Cary, NC
López-Rull I, Gil D (2009a) Elevated testosterone levels affect female breeding success and yolk androgen deposition in a passerine bird. Behav Process 82:312–318
López-Rull I, Gil D (2009b) Do female spotless starlings Sturnus unicolor adjust maternal investment according to male attractiveness? J Avian Biol 40:254–262
Lozano GA (1994) Carotenoids, parasites, and sexual selection. Oikos 70:309–311
Magrath MJL, Komdeur J (2003) Is male care compromised by additional mating opportunity? Trends Ecol Evol 18:424–430
McGraw KJ (2006) Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ (eds) Bird coloration: I. Mechanisms and measurements. Harvard University Press, Cambridge, pp 177–242
Møller AP, Biard C, Blount JD, Houston DC, Ninni P, Saino N, Surai PF (2000) Carotenoid dependent signals: indicators of foraging efficiency, immunocompetence, or detoxification ability? Avian Poultry Biol Rev 11:137–159
Morales J, Alonso-Alvarez C, Pérez C, Torres R, Serafino E, Velando A (2009) Families on the spot: sexual signals influence parent-offspring interactions. Proc R Soc Lond B 276:2477–2483
Moreno-Rueda G (2007) Yolk androgen deposition as a female tactic to manipulate paternal contribution. Behav Ecol 18:496–498
Mougeot F, Pérez-Rodríguez L, Sumozas N, Terraube J (2009) Parasites, condition, immune responsiveness and carotenoid-based ornamentation in male red-legged partridge: Alectoris rufa. J Avian Biol 40:67–74
Müller W, Eising CM, Dijkstra C, Groothuis TGG (2004) Within-clutch patterns of yolk testosterone vary with the onset of incubation in black-headed gulls. Behav Ecol 15:893–897
Müller W, Lessells CK, Korsten P, von Engelhardt N (2007) Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am Nat 169:E84–E96
Navara KJ, Badyaev AV, Mendonca MT, Hill GE (2006a) Yolk antioxidants vary with male attractiveness and female condition in the house finch (Carpodacus mexicanus). Physiol Biochem Zool 79:1098–1105
Navara KJ, Hill GE, Mendonca MT (2006b) Yolk androgen deposition as a compensatory strategy. Behav Ecol Sociobiol 60:392–398
Pérez-Rodríguez L (2008) Carotenoid-based ornamentation as a dynamic but consistent individual trait. Behav Ecol Sociobiol 62:995–1005
Pérez-Rodríguez L (2009) Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. Bioessays 31:1116–1126
Pérez-Rodríguez L, Viñuela J (2008) Carotenoid-based bill and eye ring coloration as honest signals of condition: an experimental test in the red-legged partridge (Alectoris rufa). Naturwissenschaften 95:821–830
Pérez-Rodríguez L, Mougeot F, Alonso-Alvarez C, Blas J, Viñuela J, Bortolotti GR (2008) Cell-mediated immune activation rapidly decreases plasma carotenoids but does not affect oxidative stress in red-legged partridges (Alectoris rufa). J Exp Biol 211:2155–2161
Pérez-Rodríguez L, Mougeot F, Alonso-Alvarez C (2010) Carotenoid-based coloration predicts resistance to oxidative damage during immune challenge. J Exp Biol 213:1685–1690
Ratikainen II, Kokko H (2010) Differential allocation and compensation: who deserves the silver spoon? Behav Ecol 21:195–200
Rosen RF, Tarvin KA (2006) Sexual signals of the male American goldfinch. Ethology 112:1008–1019
Rutstein AN, Gilbert L, Slater PJB, Graves JA (2004) Mate attractiveness and primary resource allocation in the zebra finch. Anim Behav 68:1087–1094
Saks L, McGraw KJ, Horak P (2003) How feather colour reflects its carotenoid content. Funct Ecol 17:555–561
Safran RJ, Pilz KM, McGraw KJ, Correa SM, Schwabl H (2008) Are yolk androgens and carotenoids in barn swallow eggs related to parental quality? Behav Ecol Sociobiol 62:427–438
Saino N, Bertacche V, Ferrari RP, Martinelli R, Møller AP, Stradi R (2002) Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc R Soc Lond B 269:1729–1733
SAS Institute (2001) SAS/STAT software: changes and enhancements, version 8.2. SAS Publishing, North Carolina
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113
Schielzeth H, Forstmeier W (2009) Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol 20:416–420
Schwabl H (1996) Maternal testosterone in the avian egg enhances postnatal growth. Comp Biochem Physiol A 114:271–276
Sheldon B (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:398–402
Skinner AMJ, Watt PJ (2007) Strategic egg allocation in the zebra fish, Danio rerio. Behav Ecol 18:905–909
Smith HG, Härdling R (2000) Clutch size evolution under sexual conflict enhances the stability of mating systems. Proc R Soc Lond B 267:2163–2170
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Surai PF (2002) Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham
Surai PT, Speake BK (1998) Distribution of carotenoids from the yolk to the tissues of the chick embryo. J Nutr Biochem 9:645–651
Szigeti B, Torok J, Hegyi G, Rosivall B, Hargitai R, Szöllösi E, Michl G (2007) Egg quality and parental ornamentation in the blue tit Parus caeruleus. J Avian Biol 38:105–112
Thear K (1987) Incubation: a guide to hatching and rearing. Broad Leys Publishing, Essex
Trivers R (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine Press, Chicago, pp 139–179
Velando A, Beamonte-Barreiros R, Torres R (2006) Pigment-based skin colour in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149:543–552
Villafuerte R, Negro JJ (1998) Digital imaging for colour measurement in ecological research. Ecol Lett 1:151–154
von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H (1999) Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc Lond B 266:1–12
Williamson KA, Surai PF, Graves JA (2006) Yolk antioxidants and mate attractiveness in the zebra finch, pp 139–359. Funct Ecol 20:354
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214
Acknowledgements
We would like to thank Diego Gil and Judith Morales for their kind review of the first version of the manuscript and also Alba Estrada, Alberto Velando, Gabriele Sorci, Deseada and Parejo Jesús Aviles for their discussion on statistics. We also thank the associated editor, Prof. Jefferson Graves and Wolfgang Forstmeier and another anonymous referee for their constructive review, particularly on the statistical procedures. We are grateful to Carlos Cano and Francisco Pérez (Consejería de Medio Ambiente, JCCM, Spain) for the kind provision of partridges for this study and to Emiliano Sobrino, Fernando Dueñas and Luis Montó for maintenance of the partridges. Lorenzo Pérez-Rodríguez was supported by a Juan de la Cierva contract (JCI-2008-2059, Ministerio de Ciencia e Innovación-Fondo Social Europeo, Spain). Francois Mougeot was supported by an intramural research project (Ministerio de Ciencia e Innovación, Spain). Financial support was obtained from the projects PII1I09-0271-5037 and PII1C09-0128-4724 from the JCCM and CGL2009-10883-C02-02 from Ministerio de Ciencia e Innovación (Spain).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Graves
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 170 kb)
Rights and permissions
About this article
Cite this article
Alonso-Alvarez, C., Pérez-Rodríguez, L., Ferrero, M.E. et al. Adjustment of female reproductive investment according to male carotenoid-based ornamentation in a gallinaceous bird. Behav Ecol Sociobiol 66, 731–742 (2012). https://doi.org/10.1007/s00265-012-1321-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1321-8