Abstract
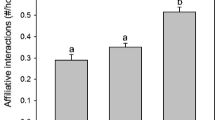
Responding differentially to kin and non-kin is known to be adaptive in many species. One example is the inclusive fitness benefits of reducing aggression toward closer relatives. Little is known, however, about the ability of animals to assess differential degrees of genetic relatedness and to respond accordingly with differential levels of aggression. In the present study, we tested whether aggressiveness between body mass-matched pairs of fire salamander (Salamandra infraimmaculata) larvae covaried with the genetic similarity between them. We quantified aggressiveness at three levels of genetic similarity by selecting pairs within and across pools from recently genotyped populations. We also assessed aggression between pairs of siblings. Aggression and associated injuries decreased as genetic similarity increased across the groups. These findings suggest that cannibalistic salamanders can assess their degree of genetic relatedness to conspecifics and vary their behavioral responses depending on the degree of similarity between them along a genetic relatedness continuum.


Similar content being viewed by others
References
Bar-David S, Segev O, Peleg N, Hill N, Templeton AR, Schultz CB, Blaustein L (2007) Long distance movements by fire salamanders (Salamandra infraimmaculata) and implications for habitat fragmentation. Isr J Ecol Evol 53:143–159
Bateson P (1983) Optimal outbreeding. In: Bateson PPG (ed) Mate choice. Cambridge University Press, Cambridge, UK, pp 257–277
Blaustein L, Friedman J, Fahima T (1996) Larval Salamandra drive temporary pool community dynamics: evidence from an artificial pool experiment. Oikos 76:392–402
Busquet N, Baudoin C (2005) Odour similarities as a basis for discriminating degrees of kinship in rodents: evidence from Mus spicilegus. Anim Behav 70:997–1002
Cohen M, Flam R, Sharon R, Ifrach H, Yeheskely-Hayon D, Warburg MR (2005) The evolutionary significance of intra-cohort cannibalism in larvae of a xeric-inhabiting salamander: an inter-cohort comparison. Curr Herpetol 24:55–66
Dawley EM (1984) Recognition of individual, sex and species odors by salamanders Plethodon glutinosus and Plethodon jordani complex. Anim Behav 32:353–361
Dawley EM (1986) The evolution of chemical signals as a premating isolating mechanism in a complex of terrestrial salamanders. In: Duvall D, Muller-Schwarze D, Silverstein RM (eds) Chemical signals in vertebrates 4. Plenum, New York, pp 241–224
Degani G (1993) Cannibalism among Salamandra salamandra larvae. Isr J Zool 39:125–129
Degani G (1996) Salamandra salamandra at the southern limit of its distribution. Laser Pages Publishing, Jerusalem, Israel
Eitam A, Blaustein L (2002) Noninvasive individual identification of larval Salamandra using tailfin spot patterns. Amphib-reptil 23:215–219
Eitam A, Blaustein L, Mangel M (2005) Density and intercohort priority effects on larval Salamandra salamandra in temporary pools. Oecologia 146:36–42
Gibbons ME, Ferguson AM, Lee DR, Jaeger RG (2003) Mother-offspring discrimination in the red-backed salamander may be context dependent. Herpetologica 59:322–333
Hamilton WD (1964) The genetical evolution of social behaviour. I and II. J Theor Biol 7:1–52
Heth G, Todrank J (2000) Individual odour similarities across species parallel phylogenetic relationships in the S. ehrenbergi superspecies of mole rats. Anim Behav 60:789–795
Heth G, Todrank J, Busquet N, Baudoin C (2003) Genetic relatedness assessment through individual odour similarities (G-ratios) in mice. Biol J Linn Soc Lond 78:595–603
Hughes J, Goudkamp K, Hurwood D, Hancock M, Bunn S (2003) Translocation causes extinction of a local population of the freshwater shrimp Paratya australiensis. Conserv Biol 17:1007–1012
Kaib M, Jmhasly P, Wilfert L, Durka W, Franke S, Francke W, Leuthold RH, Brandl R (2004) Cuticular hydrocarbons and aggression in the termite Macrotermes subhyalinus. J Chem Ecol 30:365–385
Masters BS, Forester DC (1995) Kin recognition in a brooding salamander. Proc R Soc Lond B Biol Sci 261:43–48
Peleg N (2009) Studies on the conservation of the fire salamander, Salamandra infraimmaculata, in Israel. PhD dissertation. University of Haifa, Israel
Pfennig DW (1997) Kinship and cannibalism. Bioscience 47:667–675
Pfennig DW, Collins JP (1993) Kinship affects morphogenesis in cannibalistic salamanders. Nature 362:836–838
Pfennig DW, Reeve HK, Sherman PW (1993) Kin recognition and cannibalism in spadefoot toad tadpoles. Anim Behav 46:87–94
Pfennig DW, Sherman PW, Collins JP (1994) Kin recognition and cannibalism in polymorphic salamanders. Behav Ecol 5:225–232
Reques R, Tejedo M (1996) Intraspecific aggressive behaviour in fire salamander larvae (Salamandra salamandra): the effects of density and body size. J Herpetol 6:15–19
Steinfartz S, Stemshorn K, Kuesters D, Tautz D (2006) Patterns of multiple paternity within and between annual reproduction cycles of the fire salamander (Salamandra salamandra) under natural conditions. J Zool 268:1–8
Templeton AR (1986) Coadaptation and outbreeding depression. In: Soule M (ed) Conservation biology: science of scarcity and diversity. Sinauer, Sunderland, MA, pp 105–116
Thornhill NW (ed) (1993) The natural history of inbreeding and outbreeding. Chicago Press, Chicago
Todrank J, Heth G (2003) Odor-genes covariance and genetic relatedness assessment: rethinking odor-based “recognition” mechanisms in rodents. Adv Study Behav 32:77–130
Todrank J, Busquet N, Baudoin C, Heth G (2005) Preferences of newborn mice for odours indicating closer genetic relatedness: is experience necessary? Proc R Soc Lond B Biol Sci 272:2083–2088
Tregenza T, Wedell N (2000) Genetic compatibility, mate choice and patterns of parentage: invited review. Mol Ecol 9:1013–1027
Verrell PA (1999) Geographic variation in sexual behaviour: sex, signals, and speciation. In: Foster SA, Endler JA (eds) Geographic variation in behaviour: perspectives on evolutionary mechanisms. Oxford University Press, New York, pp 262–282
Verrell PA (2003) Population and species divergence of chemical cues that influence mate recognition of females in desmognathine salamanders. Ethology 109:577–586
Walls SC, Jaeger RG (1987) Aggression and exploitation as mechanisms of competition in larval salamanders. Can J Zool 65:2938–2944
Walls SC, Roudebush RE (1991) Reduced aggression toward siblings as evidence of kin recognition in cannibalistic salamanders. Am Nat 138:1027–1038
Warburg MR (1994) Population ecology, breeding activity, longevity, and reproductive strategies of Salamandra salamandra during an 18-year long study of an isolated population on Mt. Carmel, Israel. Mertensiella 4:399–421
Acknowledgements
We thank Yoav Shulman for help in collecting and maintaining the adult females. We also thank Alan Templeton, Marc Mangel, Yoav Shulman, Asaf Sadeh, Alon Silberbush, Ori Segev, Nir Peleg, and Shirli Bar-David for fruitful discussions and R. W. Elwood and two anonymous reviewers for constructive comments on an earlier version of the manuscript. Permission to use the Salamandra was granted by the Israel Nature and Parks Authority. This study was funded by US–Israel Binational Science Foundation grant 2002-365 awarded to L. B. and Marc Mangel. The experiment complies with the current animal protection laws of Israel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Christensen-Dalsgaard
Rights and permissions
About this article
Cite this article
Markman, S., Hill, N., Todrank, J. et al. Differential aggressiveness between fire salamander (Salamandra infraimmaculata) larvae covaries with their genetic similarity. Behav Ecol Sociobiol 63, 1149–1155 (2009). https://doi.org/10.1007/s00265-009-0765-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0765-y