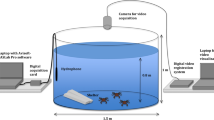
Abstract
Temperature is expected to have an effect on the behavioral patterns of all organisms, especially ectotherms. However, although several studies focused on the effect of temperature on acoustic displays in both insects and anurans, almost nothing is known about how environmental temperature may affect ectotherm visual courtship displays and sexual performance. The purpose of this study was to determine the effect of environmental temperature on the sexual behavior of Alpine newts (Triturus alpestris). We subjected T. alpestris to two different temperatures in controlled laboratory conditions. Temperature had a major effect on both male and female behaviors: at low temperature, the frequencies of several displays, including tail-raising during sperm deposition, are lowered. This variation is caused indirectly by temperature because it is due to female responsiveness, which is temperature-dependent. However, the fanning movement of the male’s tail during its main courtship display is independent of female behavior: at lower temperatures, the tail beats at a lower rate, but for a longer time. The similar reproductive success (i.e. sperm transfer) at the two temperature ranges indicates that breeding in cold water is not costly but instead allows males and females to mate early in the season. This is particularly adaptive because, in many habitats, the reproductive period is shortened by drying or freezing conditions, which may impair survival of branchiate offspring. This study also demonstrates the necessity of considering environmental parameters when modeling optimality and characteristics of ectotherm behaviors.

Similar content being viewed by others
References
Ahnesjo I (1994) Temperature affects male and female potential reproductive rates differently in the sex-role reversed pipefish, Syngnathus typhle. Behav Ecol 6:229–233
Arntzen JW, Sparreboom M (1989) A phylogeny for the old world newts, genus Triturus: biochemical and behavioral data. J Zool Lond 219:645–664
Cogalniceanu D (1994) The relative importance of vision and olfaction in mate recognition in male newts (Genus Triturus). Herpetologica 50:344–349
Connaughton MA, Fine ML, Taylor MH (2002) Weakfish sonic muscle: influence of size, temperature and season. J Exp Biol 205:2183–2188
Davenport J (1992) Animal life at low temperature. Chapman & Hall, London
Denoël M (1996) Etude comparée du comportement de cour de Triturus alpestris alpestris (Laurenti, 1768) et Triturus alpestris cyreni (Wolterstorff, 1932) (Amphibia, Caudata) : approche évolutive. Cah Ethol 16:133–258
Denoël M (1998) The modulation of movement as a behavioral adaptation to extreme environments in the newt Triturus alpestris cyreni. J Herpetol 32:623–625
Denoël M (1999) Le comportement social des urodèles. Cah Ethol 19:221–258
Denoël M (2002) Paedomorphosis in the Alpine newt (Triturus alpestris): decoupling behavioural and morphological change. Behav Ecol Sociobiol 52:394–399
Denoël M (2003) Effect of rival males on the courtship of paedomorphic and metamorphic Triturus alpestris. Copeia 2003:618–623
Denoël M, Andreone F (2003) Trophic habits and aquatic microhabitat use in gilled immature, paedomorphic and metamorphic Alpine newts (Triturus alpestris apuanus) in a pond in central Italy. Belg J Zool 133:95–102
Denoël M, Joly P (2001a) Adaptive significance of facultative paedomorphosis in Triturus alpestris (Amphibia, Caudata): resource partitioning in an alpine lake. Freshwater Biol 46:1387–1396
Denoël M, Joly P (2001b) Size-related predation reduces intramorph competition in paedomorphic Alpine newts. Can J Zool 79:943–948
Denoël M, Poncin P, Ruwet JC (2001a) Sexual compatibility between two heterochronic morphs in the Alpine newt, Triturus alpestris. Anim Behav 62:559–566
Denoël M, Poncin P, Ruwet JC (2001b) Alternative mating tactics in the Alpine newt Triturus alpestris alpestris. J Herpetol 35:62–67
Dunbar RIM (1982) Intraspecific variations in mating strategy. In: Bateson PPG, Klopfer PH (eds) Perspectives in ethology, vol 5. Plenum, New York, pp 385–431
Endler JA (1995) Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol Evol 10:22–29
Garner TWJ, Schmidt BR (2003) Relatedness, body size and paternity in the alpine newt, Triturus alpestris. Proc R Soc Lond Ser B Biol Sci 270:619–624
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. The University of Chicago Press, Chicago
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–98
Halliday TR (1977) The courtship of European newts: an evolutionary perspective. In: Taylor DH, Guttman SI (eds) The reproductive biology of amphibians. Plenum, New York, pp 185–232
Hirano M, Rome LC (1984) Jumping performance of frogs (Rana pipiens) as a function of muscle temperature. J Exp Biol 108:429–439
Houck LD, Arnold SJ (2003) Courtship and mating behavior. In: Sever DM (ed) Reproductive biology and phylogeny of Urodela. Science, Enfield, N.H., pp 383–424
Howard RD, Young JR (1998) Individual variation in male vocal traits and female mating preferences in Bufo americanus. Anim Behav 55:1165–1179
Hutchinson VH, Dupré RK (1992) Thermoregulation. In: Feder ME, Burggren WW (eds) Environmental physiology of the Amphibians. The University of Chicago Press, Chicago, pp 206–249
John-Alder HB, Barnhart MC, Bennett AF (1989) Thermal sensitivity of swimming performance and muscle contraction in northern and southern populations of tree frogs (Hyla crucifer). J Exp Biol 142:357–372
Kikuyama S, Toyoda F, Ohmiya Y, Matsuda K, Tanaka S, Hayashi H (1995) Sodefrin: a female-attracting peptide pheromone in newt cloacal glands. Science 267:1643–1645
Kissner KJ, Forbes MR (1997) Rattling behavior of prairie rattlesnakes (Crotalus viridis viridis, Viperidae) in relation to sex, reproductive status, body size, and body temperature. Ethology 103:1042–1050
Kvarnemo C (1994) Temperature differentially affects male and female reproductive rates in the sand goby: consequences for operational sex ratio. Proc R Soc Lond B 256:151–156
Kvarnemo C (1996) Temperature affects operational sex ratio and intensity of male-male competition: an experimental study of sand gobies, Pomatoschistus minutus. Behav Ecol 7:208–212
Kvarnemo C (1998) Temperature modulates competitive behaviour: why sand goby males fight more in warmer water. Ethol Ecol Evol 10:105–114
Kvarnemo C, Ahnesjö I (1996) The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408
Lass S, Spaak P (2003) Temperature effects on chemical signalling in a predator-prey system. Freshwater Biol 48:669–677
Mori A, Burghardt GM (2001) Temperature effects on anti-predator behaviour in Rhabdophis tigrinus, a snake with toxic nuchal glands. Ethology 107:795–811
Navas CA, Bevier CR (2001) Thermal dependency of calling performance in the eurythermic frog Colostethus subpunctatus. Herpetologica 57:384–395
Newman RA (1992) Adaptive plasticity in amphibian metamorphosis. Bioscience 42:671–678
Noldus (2002) The observer. Reference manual version 4.1. Noldus Information Technology, Wageningen
Rafinska A (1991) Reproductive biology of the fire-bellied toads, Bombina bombina and B. variegata (Anura: Discoglossidae): egg size, clutch size and larval period length differences. Biol J Linn Soc 43:197–210
Rafinski J, Osikowski A (2002) Sperm mixing in the Alpine newt (Triturus alpestris). Can J Zool 80:1293–1298
Rome LC, Stevens ED, John-Adler HB (1992) The influence of temperature and thermal acclimation on physiological function. In: Feder ME, Burggren WW (eds) Environmental physiology of the Amphibians. The University of Chicago Press, Chicago, pp 183–205
Ruano F, Tinaut A, Soler JJ (1999) High surface temperatures select for individual foraging in ants. Behav Ecol 11:396–404
Ryan TJ, Plague GR (2004) Hatching asynchrony, survival, and the fitness of alternative adult morphs in Ambystoma talpoideum. Oecologia 140:46–51
Schabetsberger R (1993) Der Bergmolch (Triturus alpestris, Laurenti) als Endkonsument in einem alpinen Karstsee (Dreibrüdersee, 1643 m, Totes Gebirge). PhD Thesis, University of Salzburg
Shine R, Olsson MM, Lemadter MP, Moore IT, Mason RT (2000) Effects of sex, body size, temperature, and location on the antipredator tactics of free-ranging gartersnakes (Thamnophis sirtalis, Colubridae). Behav Ecol 11:239–245
Sokal RR, Rohlf FJ (1995) Biometry. Freeman, New York
Sueur J, Sanborn AF (2003) Ambient temperature and sound power of cicada calling songs (Hemiptera: Cicadae: Tibicina). Physiol Entomol 28:340–343
Sullivan BK, Malmos KB (1994) Call variation in the Colorado river toad (Bufo alvarius): behavioral and phylogenetic implications. Herpetologica 50:146–156
Teyssedre C, Halliday T (1986) Cumulative effect of male’s displays in the sexual behaviour of the smooth newt Triturus vulgaris (Urodela, Salamandridae). Ethology 71:89–102
Vasara E, Sofianidou TS, Schneider H (1991) Bioaccoustic analysis of the yellow-bellied toad in Northern Greece (Bombina variegata scabra L., Anura, Discoglossidae). Zool Anz 226:220–236
Verrell PA (1986) Male discrimination of larger, more fecund females in the smooth newt, Triturus vulgaris. J Herpetol 20:416–422
Verrell PA (1988) Sexual interference in the Alpine newt, Triturus alpestris (Amphibia, Urodela, Salamandridae). Zool Sci 5:159–164
von Lindeiner A (1992) Untersuchungen zur Populationsökologie von Berg-, Faden- und Teichmolch (Triturus alpestris L., T. helveticus Razoumowski, T. vulgaris L.) an ausgewählten Gewässern im Naturpark Schönbuch (Tübingen). Jahrb Feldherpetol 3:1–117
Walker TJ (2000) Pulse rates in the songs of trilling field crickets (Orthoptera: Gryllidae: Gryllus). Ann Entomol Soc Am 93:565–572
Ward JV, Stanford JA (1982) Thermal responses in the evolutionary ecology of aquatic insects. Annu Rev Ecol Syst 27:97–117
Wells KD, Taigen TL, O’Brien JA (1996) The effect of temperature on calling energetics of the spring peeper (Pseudacris crucifer). Amphib Reptil 17:149–158
Whitehead PJ, Puckridge JT, Leigh CM, Seymour RS (1989) Effect of temperature on jump performance of the frog Limnodynastes tasmaniensis. Physiol Zool 62:937–949
Wong BBM, Cowling ANN, Cunningham RB, Donnelly CF, Cooper PD (2004) Do temperature and social environment interact to affect call rate in frogs (Crinia signifera)? Aust Ecol 29:209–214
Acknowledgements
We thank B. Burnside, W. Cooper and three anonymous reviewers for their constructive comments on the manuscript. Collecting permit was provided by the Ministère de la Région Wallonne (Division de la Nature et des Forêts). M. Denoël is a post-doctoral researcher at the Fonds National de la Recherche Scientifique (FNRS). This study benefitted from FNRS grants 1.5.011.03 and 1.5.120.04 (Crédit aux chercheurs).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Cooper
Rights and permissions
About this article
Cite this article
Denoël, M., Mathieu, M. & Poncin, P. Effect of water temperature on the courtship behavior of the Alpine newt Triturus alpestris. Behav Ecol Sociobiol 58, 121–127 (2005). https://doi.org/10.1007/s00265-005-0924-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0924-8