Abstract
Animals use several different types of pigments to acquire their colorful ornaments. Knowing the types of pigments that generate animal colors often provides valuable information about the costs of developing bright coloration as well as the benefits of using these signals in social or sexual contexts. It is often assumed that red, orange, and yellow colors in animals are derived from carotenoid pigments, when in fact there are other pigments that confer similar colors on animals. These include the pteridine pigments in a wide range of organisms, hemoglobin in blood-filled sinuses, the psittacofulvins of parrot feathers, and the phaeomelanin pigments in rufous or yellow feathers and fur. In this paper, we describe a quick and easy, two-step chemical method for field biologists to determine if their study species uses carotenoid pigments as integumentary colorants. This laboratory procedure first employs a thermochemical extraction technique, in which acidified pyridine is used under high temperature to free carotenoid pigments from tissue to produce a colorful, pigmented solution. Red, orange, or yellow tissues containing pteridines, hemoglobin, or eumelanins do not release colored pigments into heated pyridine. However, psittacofulvins, and occasionally phaeomelanins, will also solubilize using this method. Thus, a follow-up test is needed, using solvent transfer, to confirm the presence of carotenoids in animal tissues. The use of absorbance spectrophotometry on the colorful solution may also provide information about the predominant carotenoids that bestow color on your study animal.



Similar content being viewed by others
Notes
For certain carotenoid-enriched tissues (e.g., fruits, fish skin), other solvents, such as acetone (Grether et al. 2001) or tetrahydrofuran (McGraw et al. 2001), will also solubilize carotenoids. However, we recommend a standardized procedure that ensures that even the most tightly bound animal tissue matrices will release pigments into solution.
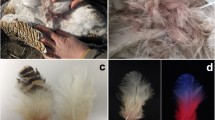
This was the case for non-carotenoid pigments extracted from chicken, turaco, swan, and eider feathers and crow eggshells. However, macaw psittacofulvins did transfer to hexane:TBME, but have never been found in animals other than parrots, and carotenoids have never been found in parrot feathers.
It is also worth noting here that lipophilic psittacofulvins and carotenoids have distinct λmax values (Hudon and Brush 1992), such that absorbance spectrophotometry can be used if necessary as a third diagnostic test to confirm the presence of carotenoids in animal tissues.
References
Bauernfeind JC (1981) Carotenoids as colorants and vitamin A precursors: technological and nutritional applications. Academic, New York
Berthold P (1967) Über Haftfarben bei Vögeln: Rostfärbung durch Eisenoxid beim Bartgeier (Gypaetus barbatus) und bei anderen Arten. Zool Jahrb Syst 93:507–595
Blair JA, Graham J (1954) The pigments of snake skins. 1. The isolation of riboflavin as a pigment of the skins of the green snakes Philothamnus semivariegatus and Dispholidus typus. Biochem J 56:286–287
Britton G (1985) General carotenoid methods. Methods Enzymol 111:507–595
Britton G, Liaaen-Jensen S, Pfander H (1995) Carotenoids. Volume 1A: isolation and analysis. Birkhäuser, Basel
Brush AH (1978) Avian pigmentation. In: Brush AH (ed) Aves, Chemical Zoology, Vol. X:141–161. Academic, New York
Brush AH (1990) Metabolism of carotenoid pigments in birds. FASEB J 4:2969–2977
Camplani A, Saino N, Møller AP (1999) Carotenoids, sexual signals and immune function in barn swallows from Chernobyl. Proc R Soc Lond B 266:1111–1116
Chen BH, Chen TM, Chien JT (1994) Kinetic model for studying the isomerization of α- and β-carotene during heating and illumination. J Agric Food Chem 42:2391–2397
Collins RP, Kalnins K, (1970) Pteridines in moth, Atteva punctella. J Insect Physiol 16:1587
Cone RD, Lu DS, Koppula S, Vage DI, Klungland H, Boston B, Chen WB, Orth DN, Pouton D, Kesterson RA (1996) The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res 51:287–318
Czeczuga B (1980a) Carotenoid content in aphids and the host plants. Bull Soc Bot Fr Actual 127:213
Czeczuga B (1980b) Investigations on carotenoids in amphibia. 2. Carotenoids occurring in various parts of the body of certain species. Comp Biochem Physiol B 65:623–630
Dale J (2000) Ornamental plumage does not signal male quality in red-billed queleas. Proc R Soc Lond B 267:2143–2149
Dyck J (1992) Reflectance spectra of plumage areas colored by green feather pigments. Auk 109:293–301
Fox D (1976) Animal biochromes and structural colors. University of California Press, Berkeley, Calif.
Fox HM, Vevers G (1960) The nature of animal colors. Macmillan, New York
Frank F (1939) Die Färbung des Vogelfeder durch Pigment und Struktur. J Ornithol 87:426–523
Goodwin TW (1984) The biochemistry of carotenoids. Volume II. Animals. Chapman and Hall, New York
Grether GF, Hudon J, Endler JA (2001) Carotenoid scarcity, synthetic pteridine pigments and the evolution of sexual coloration in guppies (Poecilia reticulata). Proc R Soc Lond B 268:1245–1253
Hata M, Hata M (1970) Carotenoid pigments in goldfish (Carassius auratus). 2. Color change and carotenoid pigment composition. Int J Biochem 2:182
Hill GE (1999) Mate choice, male quality, and carotenoid-based plumage coloration. Proc Int Ornithol Congr 22:1654–1668
Höhn EO (1955) Evidence of iron staining as the cause of rusty discoloration of normally white feathers in Anserine birds. Auk 72:414
Hudon J (1991) Unusual carotenoid use by the western tanager (Piranga ludoviciana) and its evolutionary implications. Can J Zool 69:2311–2320
Hudon J, Brush AH (1990) Carotenoids produce flush in the elegant tern plumage. Condor 92:798–801
Hudon J, Brush AH (1992) Identification of carotenoid pigments in birds. Methods Enzymol 213:312–321
Inouye CY, Hill GE, Montgomerie R, Stradi RD (2001) Carotenoid pigments in male house finch plumage in relation to age, subspecies, and ornamental coloration. Auk 118:900–915
Ito S, Fujita K (1985) Microanalysis of eumelanin and phaeomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal Biochem 144:527–536
Jørgensen K, Skibsted LH (1990) Light sensitivity of carotenoids used as food colors: quantum-yields dependence of wavelength and oxygen pressure for direct and sensitized photodegradation of solubilized lutein and β-carotene. Z Lebensm Unters Forsch 190:306–313
Katayama T, Shimaya M, Sameshim M, Chichest CO (1973) Biosynthesis of astaxanthin. 11. Carotenoids in lobster, Panulirus japonicus. Bull Jpn Soc Sci Fish 39:215–220
Kennard FH (1918) Ferruginous stains on waterfowl. Auk 35:123–132
Laruelle L, Beumont M, Legait E (1951) Recherches sur le mécanisme des changements de couleur des caroncules vasculaires du dindon (Meleagris gallopavo L.). Arch Anat Microsc Morphol Exp 40:91–113
Lubnow E (1960) Spektralphotometrische Untersuchungen an Melaninen. Zool Anz [Suppl] 23:135–139
Lucas AM, Stettenheim PR (1972) Avian anatomy. Integument. US Department of Agriculture Handbook 362, Washington, D.C.
Macedonia JM, James S, Wittle LW, Clark DL (2000) Skin pigments and coloration in the Jamaican radiation of Anolis lizards. J Herpetol 34:99–109
Massaro M, Davis LS, Darby JT (2003) Carotenoid-derived ornaments reflect parental quality in male and female yellow-eyed penguins (Megadyptes antipodes). Behav Ecol Sociobiol 55:169–175
Matsui K, Marunouchi J, Nakamura M (2002) An ultrastructural and carotenoid analysis of the red ventrum of the Japanese newt, Cynops pyrrhogaster. Pigm Cell Res 15:265–272
Mays Jr HL, McGraw KJ, Ritchison G, Rush V, Parker RS (2004) Sexual dichromatism in the yellow-breasted chat (Icteria virens): spectrophotometric analysis and biochemical basis. J Avian Biol 35:125–134
McGraw KJ (2005) Not all red, orange, and yellow colors are carotenoid-based: the need to couple biochemical and behavioral studies of color signals in birds. Proc Indian Nat Sci Acad
McGraw KJ, Hill GE (2000) Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc R Soc Lond B 267:1525–1531
McGraw KJ, Hill GE, Stradi R, Parker RS (2001) The influence of carotenoid acquisition and utilization on the maintenance of species-typical plumage pigmentation in male American goldfinches (Carduelis tristis) and northern cardinals (Cardinalis cardinalis). Physiol Biochem Zool 74:843–852
McGraw KJ, Hill GE, Stradi R, Parker RS (2002a) The effect of dietary carotenoid access on sexual dichromatism and plumage pigment composition in the American goldfinch. Comp Biochem Physiol B 131:261–269
McGraw KJ, Adkins-Regan E, Parker RS (2002b) Anhydrolutein in the zebra finch: a new, metabolically derived carotenoid in birds. Comp Biochem Physiol B 132:813–820
McGraw KJ, Hill GE, Parker RS (2003a) Carotenoid pigments in a mutant cardinal: implications for the genetic and enzymatic control mechanisms of carotenoid metabolism in birds. Condor 105:587–592
McGraw KJ, Beebee MD, Hill GE, Parker RS (2003b) Lutein-based plumage coloration in songbirds is a consequence of selective pigment incorporation into feathers. Comp Biochem Physiol B 135:689–696
McGraw KJ, Wakamatsu K, Ito S, Nolan PM, Jouventin P, Dobson FS, Austic RE, Safran RJ, Siefferman LM, Hill GE, Parker RS (2004a) You can’t judge a pigment by its color: carotenoid and melanin content of brown and yellow feathers in swallows, bluebirds, penguins, and domestic chickens. Condor 106:390–395
McGraw KJ, Safran RJ, Evans MR, Wakamatsu K (2004b) European barn swallows use melanin pigments to color their feathers brown. Behav Ecol 15:889–891
McGraw KJ, Wakamatsu K, Clark AB, Yasukawa K (2004c) Red-winged blackbirds Agelaius phoeniceus use carotenoid and melanin pigments to color their epaulets. J Avian Biol
Miksik I, Holan V, Deyl Z (1996) Avian eggshell pigments and their variability. Comp Biochem Physiol B 113:607–612
Miltenberger RJ, Mynatt RL, Bruce BD, Wilkison WO, Woychik RP, Michaud EJ (1999) An agouti mutation lacking the basic domain induces yellow pigmentation but not obesity in transgenic mice. Proc Natl Acad Sci USA 96:8579–8584
Møller AP, Biard C, Blount JD, Houston DC, Ninni P, Saino N, Surai PF (2000) Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence, or detoxification ability? Avian Poult Biol Rev 11:137–159
Moreau RE (1958) Some aspects of the Musophagidae. Part 3. Ibis 100:238–270
Negro JJ, Margalida A, Hiraldo F, Heredia R (1999) The function of the cosmetic coloration of bearded vultures: when art imitates life. Anim Behav 58:F14–F17
Oehme H (1969) Vergleichende Untersuchungen über die Färbung der Vogeliris. Biol Zentralbl 88:3–35
Oliphant LW, Hudon J, Bagnara JT (1992) Pigment cell refugia in homeotherms—The unique evolutionary position of the iris. Pigm Cell Res 5:367–371
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
Owens IPF, Hartley IR (1999) Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proc R Soc Lond B 265:397–407
Rempeters G, Henze M, Anders F (1981) Carotenoids and pteridines in the skin of interspecific hybrids of Xiphophorus. Comp Biochem Physiol B 69:91–98
Rodriguez-Amaya DB (1999) A guide to carotenoid analysis in foods. OMNI Research and ILSI Press, Washington, D.C.
Stradi R (1998) The colour of flight: carotenoids in bird plumage. Solei Gruppo Editoriale Informatico, Milan, Italy
Stradi R, Pini E, Celetano G (2001) The chemical structure of the pigments in Ara macao plumage. Comp Biochem Physiol B 130:57–63
Stresemann E (1927–1934) Aves.-Vögel. In: Kükenthal W, Krumbach T (eds) Handbuch der Zoologie, vol 7, part 2. de Gruyter, Berlin
Valadon LRG, Mummery RS (1973) Comparative study of carotenoids of ladybirds (ladybugs) milking aphids feeding on vetch. Comp Biochem Physiol 46:427–434
Völker O (1934) Die Abhängigkeit der Lipochrombildung bei Vögeln von pflanzlichen Carotinoiden. J Ornithol 82:439–450
Völker O (1936) Ueber den gelben Federfarbstoff des Wellensittichs (Melanopsittacus undulatus, Shaw). J Ornithol 84:618–630
Völker O (1937) Ueber Fluoreszierende, gelbe Federpigmente bei Papageien, eine neue Klasse von Federfarbstoffen. J Ornithol 85:136–146
Völker O (1938) Porphyrin in Vogelfedern. J Ornithol 86:436–456
With TK (1973) Porphyrins in egg shells. Biochem J 137:597–598
Wolf TM, Wasserstein R, Yoon JS (1987) Qualitative and quantitative studies of pteridine eye pigments in Drosophila. - 7 species of Hawaiian Drosophila and Drosophila melanogaster. Biochem Syst Ecol 15:239–245
Yamada S, Tanaka Y, Sameshima M, Ito Y (1990) Pigmentation of prawn (Penaeus japonicus) with carotenoids. 1. Effect of dietary astaxanthin, beta-carotene and canthaxanthin on pigmentation. Aquaculture 87:323–330
Yamaguchi K, Miki W, Toriu N, Kondo Y, Murakami M, Konosu S, Satake M, Fujita T (1983) Chemistry and utilization of plankton. 1. The composition of carotenoid-pigments in the Antarctic krill Euphausia superba. Bull Jpn Soc Sci Fish 49:1411–1415
Ziegler I (1965) Pterine als Wirkstoffe und Pigmente. Ergeb Physiol 56:1–66
Acknowledgements
This paper evolved from several inquiries and discussions at the 9th International Behavioral Ecology Congress in Montreal, Quebec, Canada. The Institutional Animal Care and Use Committee at Cornell University approved all procedures reported in our study (protocol no. 99–89). We thank T. Czeschlik and two anonymous referees for helpful comments on the manuscript. This research was funded by the Environmental Protection Agency (STAR fellowship to K.J.M.); during manuscript preparation, K.J.M. was supported by the United States Department of Agriculture (grant to K. Klasing) and by the College of Liberal Arts and Sciences and the School of Life Sciences at Arizona State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGraw, K.J., Hudon, J., Hill, G.E. et al. A simple and inexpensive chemical test for behavioral ecologists to determine the presence of carotenoid pigments in animal tissues. Behav Ecol Sociobiol 57, 391–397 (2005). https://doi.org/10.1007/s00265-004-0853-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0853-y