Abstract
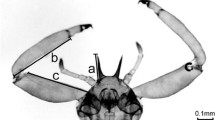
The essence of eusociality is a trade-off between producing one’s own offspring and helping collateral kin via such activities as defence, foraging and brood rearing. This trade-off often involves morphological differences between “helper” and “reproductive” castes, but the advantages, correlates and phylogenetic context of morphological caste differentiation have seldom been analysed. Six species of Australian gall-inducing thrips on Acacia show morphological polymorphism. One morph, referred to as a soldier, has reduced wings and antennae but greatly enlarged fore-femora, which are thought to be adaptations for gall defence. The other, dispersing morph, has fully developed wings and relatively slight fore-femora. Here, we quantify the defensive behaviour of soldier morphs, and compare soldier and foundresses, using behavioural assays designed to measure proclivity to attack kleptoparasites (specialised invaders in the genus Koptothrips) and effectiveness in killing them. In all five species investigated, soldiers were able to kill Koptothrips. Moreover, the effectiveness of soldiers was relatively high in the more-derived species in the phylogeny of the clade, Kladothrips intermedius, K. habrus and K. waterhousei. Soldiers of K. intermedius and K. habrus also killed kleptoparasites more effectively than did foundresses, and K. habrus soldiers exhibited higher proclivity to attack than did foundresses. Data from naturally invaded galls demonstrate that soldiers in the field do kill Koptothrips, and vice versa. These results show that soldiers of Australian gall thrips are motivated and effective for gall defence.


Similar content being viewed by others
References
Ananthakrishnan TN (1992) Unique aspects in the biology of thrips-induced galls. In: Shorthouse JD, Rohfritsch O (eds) Biology of insect induced galls. Oxford University Press, New York, pp 185–195
Bejah K (1997) The functional significance of morphological and behavioural castes in Oncothrips tepperi in galls on Acacia oswaldii. Thesis, Flinders University of South Australia
Bourke AFG (1999) Colony size, social complexity and reproductive conflict in social insects. J Evol Biol 12:245–257
Bourke AFG, Franks N (1995) Social evolution in ants. Princeton University Press, Princeton
Chapman TW, Crespi BJ (1998) High relatedness and inbreeding in two species of haplodiploid eusocial thrips (Insecta: Thysanoptera) revealed by microsatellite analysis. Behav Ecol Sociobiol 43:301–306
Chapman TW, Crespi BJ, Schwarz MP, Kranz, BD (2000) High relatedness and inbreeding at the origin of eusociality in Australian gall thrips. PNAS 97:1648–1650
Chapman TW, Kranz BD, Bejah K, Morris DC, Schwarz MP, Crespi BJ (2002) The evolution of soldier reproduction in social thrips. Behav Ecol 13:519–525
Crespi BJ (1992a) Eusociality in Australian gall thrips. Nature 359:724–726
Crespi BJ (1992b) The behavioral ecology of Australian gall thrips. J Nat Hist 26:769–809
Crespi BJ, Abbot P (1999) The behavioral ecology and evolution of kleptoparasitism in Australian gall thrips. Fla Entomol 82:147–164
Crespi BJ, Choe JC (1997) Evolution and explanation of social systems. In: Choe JC, Crespi BJ (eds) The evolution of social behaviour in insects and arachnids. University Press, Cambridge, pp 499–524
Crespi BJ, Mound LA (1997) Ecology and evolution of social behaviour among Australian gall thrips and their allies. In: Choe JC, Crespi BJ (eds) The evolution of social behaviour of insects and arachnids. Cambridge University Press, Cambridge, pp 166–180
Crespi BJ, Worobey M (1998) Comparative analysis of gall morphology in Australian gall thrips: the evolution of extended phenotypes. Evolution 52:1686–1696
Crespi BJ, Yanega D (1995) The definition of eusociality. Behav Ecol 6:109–115
Crespi BJ, Carmean D, Chapman TW (1997a) The ecology and evolution of galling thrips and their allies. Annu Rev Entomol 42:51–71
Crespi BJ, Carmean D, Mound LA, Worobey M, Morris DC (1997b) Phylogenetics of social evolution in Australian gall-forming thrips: evidence from mitochondrial DNA sequence, adult morphology, and gall morphology. Mol Phylogenet Evol 9:163–180
Crespi BJ, Morris DC, Mound LA (2004) Evolution of ecological and behavioural diversity: Australian Acacia thrips as model organisms. Australian Biological Resources Study and Australian National Insect Collection. CSIRO Entomology, Canberra
Foster WA (1990) Experimental evidence for effective and altruistic colony defence against natural predators by soldiers of the gall-forming aphid Pemphigus spyroythecae (Hemiptera: Pemphigidae). Behav Ecol Sociobiol 27:421–430
Foster WA, Rhoden PK (1998) Soldiers defend colonies against predators in the field. Anim Behav 55:761–765
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Cambridge, Mass
Jeon J, Choe JC (2003) Reproductive skew and the origin of sterile castes. Am Nat 161:206–224
Kranz BD, Schwarz MP, Mound LA, Crespi BJ (1999) Social biology and sex ratios of the eusocial gall-inducing thrips Kladothrips hamiltoni. Ecol Entomol 24:432–442
Kranz BD, Chapman TW, Crespi BJ, Schwarz MP (2001a) Social biology and sex ratios in the gall-inducing thrips, Oncothrips waterhousei and Oncothrips habrus. Insectes Soc 48:315–323
Kranz BD, Schwarz MP, Wills TE, Chapman TW, Morris DC, Crespi BJ (2001b) A fully reproductive fighting morph in a soldier clade of gall-inducing thrips. Behav Ecol Sociobiol 50:151–161
Krawac CA (2001) The evolution of galls size in Australian gall-inducing thrips on Acacia. Thesis, Flinders University of South Australia
Michener CD (1974) The social behaviour of the bees: a comparative study. Harvard University Press, Cambridge, Mass
Morris DC, Schwarz MP, Crespi BJ, Cooper JB (2001) Phylogenetics of gall-inducing thrips on Australian Acacia. Biol J Linn Soc 74:73–86
Mound LA (1971) Gall-forming thrips and allied species (Thysanoptera: Phlaeothripinae) from Acacia trees in Australia. Bull Br Mus Nat Hist Entomol 25:389–466
Mound LA, Crespi BJ (1995) Biosystematics of two new species of Australia Acacia gall-thrips with soldiers (Insecta; Thysanoptera). J Nat Hist 29:147–157
Mound LA, Heming BS (1991) Thysanoptera. Insects of Australia, vol 1. Commonwealth Scientific and Industrial Research Organisation, division of Entomology. Melbourne University Press, Melbourne
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Perry SP, McLeish SJ, Schwarz MP, Boyette AH, Zammit J, Chapman TW (2003) Variation in propensity to defend by reproductive gall morphs in two species of gall-forming thrips. Insectes Soc 49:1–5
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Ricklefs RE, Stark JM (1996) Applications of phylogenetically independent contrasts: a mixed progress report. Oikos 77:167–172
Shingleton AW, Foster WA (2001) Behaviour, morphology and the division of labour in two soldier-producing aphids. Anim Behav 62:671–679
Sokal RR, Rohlf JF (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Wills TE, Chapman TW, Kranz BD, Schwarz MP (2001) Reproductive division of labour coevolves with gall size in Australian thrips with soldiers. Naturwissenschaften 88:526–529
Wilson EO (1971) The insect societies. Belknap Press of Harvard University Press, Cambridge, Mass
Wilson EO (1975) Sociobiology. Belknap Press of Harvard University Press, Cambridge, Mass
Acknowledgements
This work was supported by an Australian Research Council Grant to M.P. Schwarz, J.B. Cooper, B.J. Crespi and T.W. Chapman (DP0346322) and an ARC postdoctoral fellowship to T.W. Chapman. We are grateful to Dr. Laurence Mound, Dr. Laurence Packer and anonymous reviewers for their thoughtful contributions on the drafts of the manuscript, and to Michael McLeish, John Zammit and Dr. Adam L. Cronin for their assistance during field collections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Gwynne
Rights and permissions
About this article
Cite this article
Perry, S.P., Chapman, T.W., Schwarz, M.P. et al. Proclivity and effectiveness in gall defence by soldiers in five species of gall-inducing thrips: benefits of morphological caste dimorphism in two species (Kladothrips intermedius and K. habrus). Behav Ecol Sociobiol 56, 602–610 (2004). https://doi.org/10.1007/s00265-004-0811-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-004-0811-8