Abstract
Purpose
Extended reality (XR) is defined as a spectrum of technologies that range from purely virtual environments to enhanced real-world environments. In the past two decades, XR-assisted surgery has seen an increase in its use and also in research and development. This scoping review aims to map out the historical trends in these technologies and their future prospects, with an emphasis on the reported outcomes and ethical considerations on the use of these technologies.
Methods
A systematic search of PubMed, Scopus, and Embase for literature related to XR-assisted surgery and telesurgery was performed using Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) guidelines. Primary studies, peer-reviewed articles that described procedures performed by surgeons on human subjects and cadavers, as well as studies describing general surgical education, were included. Non-surgical procedures, bedside procedures, veterinary procedures, procedures performed by medical students, and review articles were excluded.
Studies were classified into the following categories: impact on surgery (pre-operative planning and intra-operative navigation/guidance), impact on the patient (pain and anxiety), and impact on the surgeon (surgical training and surgeon confidence).
Results
One hundred and sixty-eight studies were included for analysis. Thirty-one studies investigated the use of XR for pre-operative planning concluded that virtual reality (VR) enhanced the surgeon’s spatial awareness of important anatomical landmarks. This leads to shorter operating sessions and decreases surgical insult. Forty-nine studies explored the use of XR for intra-operative planning. They noted that augmented reality (AR) headsets highlight key landmarks, as well as important structures to avoid, which lowers the chance of accidental surgical trauma. Eleven studies investigated patients’ pain and noted that VR is able to generate a meditative state. This is beneficial for patients, as it reduces the need for analgesics. Ten studies commented on patient anxiety, suggesting that VR is unsuccessful at altering patients’ physiological parameters such as mean arterial blood pressure or cortisol levels. Sixty studies investigated surgical training whilst seven studies suggested that the use of XR-assisted technology increased surgeon confidence.
Conclusion
The growth of XR-assisted surgery is driven by advances in hardware and software. Whilst augmented virtuality and mixed reality are underexplored, the use of VR is growing especially in the fields of surgical training and pre-operative planning. Real-time intra-operative guidance is key for surgical precision, which is being supplemented with AR technology. XR-assisted surgery is likely to undertake a greater role in the near future, given the effect of COVID-19 limiting physical presence and the increasing complexity of surgical procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern surgery is enhanced by digital technologies which aim to improve the safety and effectiveness of procedures. These technologies include patient-specific 3D surgical planning, simulation training, navigated and robotic tools, remote assistance, and the augmentation of the operative scene with holograms [1, 2].
Extended reality (XR) describes the display of computer-generated images (CGI) in the wearer’s field of view and is a spectrum from real life (no digital augmentation) to virtual reality (VR), whereby the user’s view of the real world is occluded (Fig. 1). VR was first described in a fictional story by Stanley Weinbaum in 1935 [3], and since then, components of XR have evolved to include augmented reality (AR) and augmented virtuality (AV) (Fig. 2). XR platforms have been utilised in many aspects of surgery: pre-operative planning, surgical training, intra-operative visualisation, and guidance, particularly for navigation around complex obscure anatomy [4]. Global restrictions in working hours and increased scrutiny of patient outcomes have improved patient safety but have reduced the exposure of surgical trainees to the volume and breadth of cases. COVID-19 has had an additional detrimental impact on surgical training, with trainees performing up to 50% fewer procedures as the primary surgeon [4], while experienced surgeons have had reduced exposure to even routine surgery and risk skill disuse [5]. In high-income countries, complex surgery is typically centralised, but in the resource-poor setting or where surgeons feel “out of practice”, XR may offer a critical layer of support and safety. For the patient, XR may have a role in improving familiarity with the upcoming procedure, facilitating better consent, and reducing peri-operative anxiety.
Given the increasing role that XR has in surgery and the growing volume of literature in this field, the aim of this scoping review was four-fold:
-
Identify the type of XR most frequently used in various surgical specialities and phases of surgical intervention.
-
Identify key outcome measures and trends for the use of XR in surgery.
-
Determine if XR has been a promising addition to surgery, and which aspect of surgical practice has benefited the most.
-
Identify opportunities and challenges for XR development and usage in the future.
Methodology
This review was conducted based on the PRISMA extension for scoping reviews (PRISMA-ScR) guidelines [6] and Arksey and O’Malley’s 5-stage methodological framework for scoping reviews [7].
A systematic search from inception to November 25, 2020, was performed on three databases: PubMed MEDLINE, Ovid EMBASE, and Scopus. The search included variations of the following terms: surgery, outcomes, complications, augmented reality, mixed reality, virtual reality, and extended reality; the full search strategy is shown in Supplementary Table 1. Inclusion and exclusion criteria were determined using the PICOS model (population, intervention, comparison, outcome, study type) [8], as shown in Table 1. Two reviewers independently screened studies based on the inclusion and exclusion criteria. Initial screening consisted of title and abstract only, followed by a full-text review. At all stages, reasons for exclusion were documented. The senior author was contacted in case of discrepancy.
Following full-text review, included studies were classified into three categories: impact on surgery with two subgroups (pre-operative planning and intra-operative navigation/guidance), impact on the patient with two subgroups (pain and anxiety/understanding), and impact on the surgeon with two subgroups (surgical training and surgeon confidence).
Results
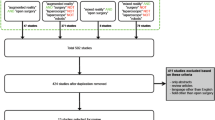
A total of 2391 articles were identified after the initial search from three databases. After deduplication, 1231 articles remained for the title and abstract screening, from which 238 full-text studies were reviewed. In total, 168 articles were eligible for inclusion (Supplementary Table 2). Publication dates ranged from 1998 to 2020. Figure 3 presents the PRISMA diagram.
Impact on surgery
Pre-operative planning
Thirty-one papers focused on pre-operative planning. Immersive VR systems improved the surgeon’s understanding of key anatomical sites [9] and improved the localisation of key areas of lesions that need to be resected [10], which are sometimes hidden from the surgeon’s view. This led to a reduced operative time, reduced damage to the neighbouring tissue, reduced blood loss, and a shorter hospital stay [11]. Furthermore, AR is more accurate in identifying anatomical variation between patients, which can be missed on a CT scan, as AR correlates better with patient anatomy than CT scans alone [12]. VR accurately facilitated the pre-operative reconstruction of sophisticated vasculature and improved the spatial understanding of vascular anatomy, allowing safe vessel control during surgery [13]. During tumour resection, it is crucial to demarcate the boundary between the tumour and healthy tissue/vasculature; VR improves the accuracy of this process [14].
Intra-operative
Forty-nine papers were included in the category of intra-operative guidance and imaging for surgery. With increasing computing power and available hardware, such as Microsoft HoloLens and iPads [15], an increasing numbers of studies explored the feasibility of XR, especially AR, for intra-operative usage. Traditionally, a tri-panel display conveyed visual information to surgeons during operations; however, viewing images required looking away from the operative site. With the development of AR headsets, surgeons do not need to break their line of sight [16], with improved depth perception of fine structures [17] and motion parallax [18]. Real-time intra-operative guidance is key to defining complex structures, allowing greater surgical precision and better placement of surgical devices [12].
Intra-operative XR guidance has been well explored; thus, larger clinical trials with longer follow-up times were reported, ranging from 2 to 12 months [19,20,21]. Studies reporting XR usage for other surgical phases had follow-up time shorter than a patient’s hospital stay. Specialities that utilised intra-operative XR guidance the most are neurosurgery [22] and orthopaedics [23].
AR intra-operative usage involves a scan of internal structures pre-operatively using CT [24] or MRI [25]. The scan was then coupled with external marker reference points attached to the patient’s skin, providing an anchor point for hologram projections such that even with small incisions, internal structures can be visualized as an overlay image on the surface. In addition, modifications can be made that highlight “negative structures”, so that they are not accidentally incised [26], an important feature for novice surgeons. Disadvantages of this method included cables cluttering operative space [16], high additional upfront cost [27], and calibration of internal and external spaces being inconsistent [28]. Two studies reported no differences between traditional display methods and AR-assisted methods [29, 30]. One study even concluded that AR-assisted surgery is inferior in key aspects to the default [31].
Impact on the patient
Pain
Eleven studies describe how XR can modulate pain for patients. XR has been used to supplement sedative and pain relief processes prior to [32], during [33], and after surgery [34]. VR has been the most prevalent form of XR used, inducing a meditative state during or after the surgery.
Usually, a VR headset, such as the Microsoft Oculus Rift, was given to the patient, with which they could try to catch imaginary creatures in the virtual realm [34], relax on a beach [35], or adventure through the Arctic tundra [36]. Outcomes of such clinical trials were compared to a control that uses conventional sedative medication. Physiological outcomes reported that less sedative is required for patients to feel at ease, increasing patient satisfaction and the desire to use it in the future [37]. This reduced patient medication, lessened side effects, and engaged the patient more in healthcare provision.
Results from the literature were heterogeneous; one study suggested no effects on pain [32], and three studies reported no change in intra-operative pain, but a reduction in sedatives was required [35, 38, 39]. Post-operatively, VR helped with pain reduction during rehabilitation and gave the patient more confidence and independence [40]. No studies have determined XR’s intra-operative effects on patients’ long-term well-being.
Anxiety reduction
Ten studies identified methods for anxiety reduction. Traditionally, information leaflets and conversions with the surgical team were used for patient understanding prior to surgery. XR provides a novel alternative to minimise patient anxiety, with VR being the most studied. Increasing patient understanding and diminishing anxiety ameliorates the patient experience, allowing for better cooperation.
Anxiety reduction can be split into pre-operative, intra-operative, and post-operative stages. The majority of pilot interventions were at the pre-operative stage [39, 41, 42]. Similar to pain management, meditation and exploration modules exist [41, 42]. This involved the simulation of surgical procedures or operation room tours (usually for paediatric cases) [39]. Outcomes measured include standardised scores of anxiety, such as the Hospital Anxiety and Depression Scale (HDAS), Likert type scale, visual analogue scale for anxiety (VAS-A), and modified Yale pre-operative anxiety scale (m-YPAS) [43, 44].
Some papers reported patients’ self-reported feelings as well as physiological parameters such as breathing rate, mean arterial blood pressure, heart rate, and salivary cortisol [45].
Overall, whilst VR lowered patients’ anxiety and apprehension, it did not have a significant effect on objective measurements of patients’ physiological conditions [39].
Impact on the surgeon
Surgical training
Surgical training was studied in sixty papers. Surgical training has traditionally been achieved by observation and apprenticeships in operating theatres. Due to the increasing parallel duties of surgical trainees/residents, time and experience in the operating theatre come at a premium. Concern about patient safety and a reduction in working hours, coupled with the steeper learning curve of complex modern laparoscopic and arthroscopic procedures, have made surgical training an area requiring innovation [46, 47].
The most cost-effective and efficient training method has been VR; this can be done as a standalone procedure with few external inputs. AR training programs also exist, such as dentistry, whereby the AR headset interacts with a 3D model, with the trainee using live tools monitored electronically. Specialities with frequent VR usage were variations in laparoscopic procedures [48] and orthopaedic procedures [49].
The time frame of VR training sessions is usually a few months. Surgical simulators can be divided into two groups: basic isolated skills trainers versus specific procedural task trainers [50] and low-fidelity versus high-fidelity simulators [51]. Outcome measures seen in the literature included an overall judgement of skills by experts in the field and measurements of objective performance using procedure-specific scores [52]. Quantitative metrics were commonplace, such as time of surgery [53], complication rate [54], tool path length/tissue protection [55], degree of hand/head movement [56], and self-reported feelings [57].
Two studies suggested that these training programs increase surgical trainees’ competence when they perform their first surgery on a real patient. One study reported on the total cost of developing their own simulators, estimated at $4000–$8000 [58].
The literature included four main ways to assess training programs: construct validity (the ability to measure what it claims to be measuring), face validity (whether the program is suitable for its aims), content validity (the degree to which it is fully representative of what it aims to measure), and concurrent validity (how well a test performs compared to its well-established predecessors). Construct validity was assessed by comparing objective outcome metrics between a spectrum of surgeons on different sections of the learning curve [59]. Outcomes that determine their score were the time of surgery, tool path length, and surgical errors. A statistically significant difference indicates this program can stratify surgeons according to skill level.
Other studies attempted to convert the program into a test of skills, by either using a large number of surgeons’ performance in the program as a benchmarking distribution [56], using experts’ program performance as the benchmark [60], or correlating program performance with a real surgical performance by the same participant [61]. The latter may have ethical implications, since non-experienced surgeons are allowed to independently operate on patients in order to calibrate the scale’s extreme ends. Inter-procedural calibration was also achieved by correlating scores of different procedures in the same field [59]. Face validity was demonstrated by obtaining surgeons’ views with questionnaires on a range of aspects after they have used the program [62]. The majority of studies reported their training and assessment programs to be valid for their purposes.
Surgeon confidence
Seven papers documented surgeon confidence. The majority focused on VR-assisted “warm up” tasks prior to surgery [63], as well as pre-operative planning [64]. There was a significant increase in subjective confidence and improved objective parameters in the “warmed up” group, although the effects are diminished if the VR task was not done immediately before surgery [65]. VR’s beneficial effects were augmented for senior surgeons, for more complex procedures, and when there was real-time feedback during the “warm up” from more experienced surgeons.
Discussion
This scoping review showed the wide range of specialities and roles in which XR has been deployed to improve surgery. There has been an exponential increase in studies demonstrating the development and deployment of XR—in particular, VR and AR—for surgical training, pre-operative planning, intra-operative surgical performance, and to improve patients’ experience of surgery. This is likely driven by advances in the capability and affordability of XR headsets in the last decade and the increasing acceptance of these technologies by surgeons and patients.
The main themes identified are pre-operative planning, intra-operative guidance/imaging, and surgical training. Regarding the type of XR used, surgical training was mostly assisted by VR and intra-operative guidance by AR. The other sections of the XR spectrum, namely augmented virtuality and mixed reality, are relatively underexplored. In augmented virtuality, a real-world object is directly added into a simulated environment, and whilst it is able to isolate the issue in a system of simulated environments, often the entire patient is taken into account when planning procedures. Thus, often, augmented virtuality will be replaced by either augmented reality or virtual reality. The definition of mixed reality is still ambiguous in the literature and was mentioned once in one study [66]. Figure 4 summarises how XR has been integrated into different phases of surgery, organised by the impact on the surgeon and the surgical procedure, or the patient. Figure 5 depicts the various outcome measures reported in the literature on XR-assisted surgery.
The highest quality evidence for XR-assisted surgery was identified in relation to surgical training. Having surgeons train in simulators that allow them to virtually practice with the same tools which they later use in real surgery diminished unfamiliarity, removed part of the learning curve from the real operating room, and enabled the measurement of acceptable levels of competency. This could streamline the selection, training, and certification processes.
XR (specifically AR) has been developed more for intra-operative assistance in open procedures rather than endoscopic surgery. This may change in the coming decade, as several studies have demonstrated that 3D displays may result in better accuracy, faster surgery, and fewer errors in endoscopic surgery [67,68,69]. Thus far, VR surgical training had been most utilised for endoscopic procedures, and in particular for procedures that have longer learning curves such as hip arthroscopy and mastoid surgery [56, 70, 71]. However, a number of recent trials have demonstrated the positive impact of immersive VR [49, 52, 58, 72,73,74,75,76] and AR for surgical training for open procedures [59, 77]. Again, the balance between XR for endoscopic versus open surgery simulation is likely to shift in the coming decade with the advent of affordable and effective iVR training platforms.
With the exception of a number of studies evaluating XR-assisted intra-operative guidance, there was a paucity of studies directly comparing these technologies to a control group [29, 31, 78, 79]. The few studies that undertook direct comparisons often showed insignificant differences. This could be due to publication bias, since the results of smaller-scale studies investigating novel devices are likely to be shared, whereas real-world testing of novel technologies is complex, and many factors other than the use of the XR intervention can impact outcomes. Instead, these studies demonstrate the safety and equivalence of the technologies [16, 17, 26, 80,81,82] and large studies and long-term data are required to adequately examine their impact.
The follow-up time in most studies was short, with most outcomes reported either intra- or immediately post-operatively. However, some surgeon-oriented studies in this area investigated long-term career progression and outcomes of XR-assisted training and assessment [83].
Since the start of the twenty-first century, the literature has shown two major periods of advancement: the 2000s were focused on VR for endoscopic simulation, telesurgery trials, and proof of concept studies in stereoscopic displays, holograms, and other guises of AR. The development, validation, and deployment of XR have dominated the literature since 2010, with particular advancements in AR and immersive VR headsets. We did not identify any data investigating patients’ views on XR-assisted surgery. Instead, there has been a greater focus on the cost, reliability, and feasibility of these technologies to establish their place in surgery, when compared to studies published in the early 2000s [84]. The patient viewpoint is of particular relevance due to increased international scrutiny on how patient’s data is used and how their privacy is protected and requires further research.
We propose three main areas of research that future studies could explore:
-
1.
Conducting large-scale studies with adequately designed control arms will provide more statistical power to any differences in outcomes between novel technologies and the status quo. With the exception of intra-operative XR assistance, these are lacking in the literature.
-
2.
Patient’s views regarding the new technologies should be collated and reported. Currently, only those directly impacting the patient, such as pain/anxiety reduction, are qualitatively analysed. Patients should have the opportunity to express their views on all aspects of their surgical journey.
-
3.
Longer follow-up times are needed in studies to investigate the long-term career impacts on surgeons and the long-term impacts on patients’ quality of life.
Limitations
A scoping review is a novel clinical research method for mapping out the literature in a novel and rapidly advancing research field like XR-assisted surgery, in order to suggest gaps in the literature for future primary studies and systematic reviews. The inclusion criteria were limited to primary studies involving surgical personnel, meaning that novel theoretical papers were missed, as well as trials involving the general public. Nevertheless, these limitations were necessary to avoid commenting on hypotheticals that may not translate to clinical practice. Similarly, the exclusion of grey literature and non-English papers was a decision made to focus on well-established studies published in international journals. In such a rapidly growing field, some novel research may have occurred since the time we performed our literature search; hence, relevant studies may have been missed in the intervening time.
Conclusions
This scoping review provides an overview of XR-assisted surgery, detailing its history and developments in the past two decades, with an emphasis on surgical specialties, outcome measures, and the utility of the technologies for the surgeon and patient. Currently, the most studied areas of XR-assisted surgery are surgical training, pre-operative preparation, and intra-operative guidance and imaging. Training and pre-operative preparation are mainly achieved by virtual reality, whereas intra-operative guidance is supplemented mainly with augmented reality.
In the past decade, XR-assisted surgery has seen significant growth, fuelled by technological advances in hardware and software. As XR is now affordable, usable, acceptable, and increasingly well-validated, we recommend future studies focus on improving methodological rigour and longer follow-up, with a new focus on understanding patients’ views of these novel technologies.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Code availability
Not applicable.
References
Thimbleby H (2013) Technology and the future of healthcare. J Public health Res 2(3):28. https://doi.org/10.4081/jphr.2013.e28
Alotaibi YK, Federico F (2017) The impact of health information technology on patient safety. Saudi Med J 38(12):1173–1180. https://doi.org/10.15537/smj.2017.12.20631
Andrews C, Southworth MK, Silva JNAJR, Silva JNAJR (2019) Extended reality in medical practice. Curr Treat Options Cardiovasc Med 21(4):18. https://doi.org/10.1007/s11936-019-0722-7
Munro C, Burke J, Allum W, Mortensen N (2021) Covid-19 leaves surgical training in crisis. BMJ (Clinical research ed.) 372:n659. https://doi.org/10.1136/bmj.n659
Hughes R, Hallstrom B, Schemanske C, Howard PW, Wilton T (2020) Returning to operating following COVID-19 shutdown: what can human factors tell us? Bone Joint J 102(10):1277–1278. https://doi.org/10.1302/0301-620X.102B10.BJJ-2020-1450.R1
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169(7):467–473. https://doi.org/10.7326/M18-0850
Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract 8(1):19–32. https://doi.org/10.1080/1364557032000119616
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S (2014) PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 14: 579. https://doi.org/10.1186/s12913-014-0579-0
Yan EG, Rennert RC, Levy DM, Levy ML (2021) Three-dimensional modeling of complex pediatric intracranial aneurysmal malformations with a virtual reality system. Simul Healthc 16(4):295–300. https://doi.org/10.1097/SIH.0000000000000498
Porpiglia F, Checcucci E, Amparore D et al (2020) Three-dimensional augmented reality robot-assisted partial nephrectomy in case of complex tumours (PADUA ≥10): a new intraoperative tool overcoming the ultrasound guidance. Eur Urol 78(2):229–238. https://doi.org/10.1016/J.EURURO.2019.11.024
Shirk JD, Thiel DD, Wallen EM et al (2019) Effect of 3-dimensional virtual reality models for surgical planning of robotic-assisted partial nephrectomy on surgical outcomes: a randomized clinical trial. JAMA Netw Open 2(9):e1911598–e1911598. https://doi.org/10.1001/JAMANETWORKOPEN.2019.11598
Alexander C, Loeb AE, Fotouhi J, Navab N, Armand M, Khanuja HS (2020) Augmented reality for acetabular component placement in direct anterior total hip arthroplasty. J Arthroplasty 35(6):1636-1641.e3. https://doi.org/10.1016/J.ARTH.2020.01.025
Kockro RA, Killeen T, Ayyad A et al (2016) Aneurysm surgery with preoperative three-dimensional planning in a virtual reality environment: technique and outcome analysis. World Neurosurg 96:489–499. https://doi.org/10.1016/J.WNEU.2016.08.124
Qiu TM, Zhang Y, Wu JS et al (2010) Virtual reality presurgical planning for cerebral gliomas adjacent to motor pathways in an integrated 3-D stereoscopic visualization of structural MRI and DTI tractography. Acta Neurochir (Wien) 152(11):1847–1857. https://doi.org/10.1007/S00701-010-0739-X
Rassweiler JJ, Müller M, Fangerau M et al (2012) iPad-assisted percutaneous access to the kidney using marker-based navigation: initial clinical experience. Eur Urol 61(3):628–631. https://doi.org/10.1016/J.EURURO.2011.12.024
Gan A, Cohen A, Tan L (2019) Augmented reality-assisted percutaneous dilatational tracheostomy in critically Ill patients with chronic respiratory disease. J Intensive Care Med 34(2):153–155. https://doi.org/10.1177/0885066618791952
Battaglia S, Badiali G, Cercenelli L, Bortolani B, Marcelli E, Cipriani R, Contedini F, Marchetti C, Tarsitano A (2019) Combination of CAD/CAM and augmented reality in free fibula bone harvest. Plastic and reconstructive surgery. Global open 7(11):e2510. https://doi.org/10.1097/GOX.0000000000002510
Tang R, Ma L, Xiang C, Wang X, Li A, Liao H, Dong, J (2017) Augmented reality navigation in open surgery for hilar cholangiocarcinoma resection with hemihepatectomy using video-based in situ three-dimensional anatomical modeling: a case report. Medicine 96(37):e8083. https://doi.org/10.1097/MD.0000000000008083
Puliatti S, Sighinolfi MC, Rocco B et al (2019) First live case of augmented reality robot-assisted radical prostatectomy from 3D magnetic resonance imaging reconstruction integrated with PRECE model (predicting extracapsular extension of prostate cancer). Urol Video J 1:100002. https://doi.org/10.1016/J.UROLVJ.2019.100002
Tan T, Behary P, Tharakan G et al (2017) The effect of a subcutaneous infusion of GLP-1, OXM, and PYY on energy intake and expenditure in obese volunteers. J Clin Endocrinol Metab 102(7):2364–2372. https://doi.org/10.1210/jc.2017-00469
Wei P, Yao Q, Xu Y, Zhang H, Gu Y, Wang L (2019) Percutaneous kyphoplasty assisted with/without mixed reality technology in treatment of OVCF with IVC: a prospective study. J Orthop Surg Res 14(1):1–9. https://doi.org/10.1186/S13018-019-1303-X/TABLES/3
Auloge P, Cazzato RL, Ramamurthy N et al (2020) Augmented reality and artificial intelligence-based navigation during percutaneous vertebroplasty: a pilot randomised clinical trial. Eur Spine J 29(7):1580–1589. https://doi.org/10.1007/S00586-019-06054-6
Edström E, Burström G, Persson O et al (2020) Does augmented reality navigation increase pedicle screw density compared to free-hand technique in deformity surgery? Single surgeon case series of 44 patients. Spine (Phila Pa 1976) 45(17):E1085–E1090. https://doi.org/10.1097/BRS.0000000000003518
Liu H, Wu J, Tang Y et al (2019) Percutaneous placement of lumbar pedicle screws via intraoperative CT image-based augmented reality-guided technology. J Neurosurg Spine 32(4):542–547. https://doi.org/10.3171/2019.10.SPINE19969
Tomikawa M, Hong J, Shiotani S et al (2010) Real-time 3-dimensional virtual reality navigation system with open MRI for breast-conserving surgery. J Am Coll Surg 210(6):927–933. https://doi.org/10.1016/J.JAMCOLLSURG.2010.01.032
Citardi MJ, Agbetoba A, Bigcas JL, Luong A (2016) Augmented reality for endoscopic sinus surgery with surgical navigation: a cadaver study. Int Forum Allergy Rhinol 6(5):523–528. https://doi.org/10.1002/ALR.21702
Borgmann H, Rodríguez Socarrás M, Salem J et al (2017) Feasibility and safety of augmented reality-assisted urological surgery using smartglass. World J Urol 35(6):967–972. https://doi.org/10.1007/S00345-016-1956-6
Shen J, Zemiti N, Taoum C et al (2020) Transrectal ultrasound image-based real-time augmented reality guidance in robot-assisted laparoscopic rectal surgery: a proof-of-concept study. Int J Comput Assist Radiol Surg 15(3):531–543. https://doi.org/10.1007/S11548-019-02100-2
Ogawa H, Kurosaka K, Sato A, Hirasawa N, Matsubara M, Tsukada S (2020) Does an augmented reality-based portable navigation system improve the accuracy of acetabular component orientation during THA? A randomized controlled trial. Clin Orthop Relat Res 478(5):935–943. https://doi.org/10.1097/CORR.0000000000001083
Pellegrino G, Mangano C, Mangano R, Ferri A, Taraschi V, Marchetti C (2019) Augmented reality for dental implantology: a pilot clinical report of two cases. BMC Oral Health 19(1):1–8. https://doi.org/10.1186/S12903-019-0853-Y/FIGURES/10
Pietruski P, Majak M, Światek-Najwer E et al (2019) Supporting mandibular resection with intraoperative navigation utilizing augmented reality technology - a proof of concept study. J Craniomaxillofac Surg 47(6):854–859. https://doi.org/10.1016/J.JCMS.2019.03.004
Ong TL, Ruppert MM, Akbar M et al (2020) Improving the intensive care patient experience with virtual reality-a feasibility study. Crit care Explor 2(6):e0122. https://doi.org/10.1097/CCE.0000000000000122
Faruki A, Nguyen T, Proeschel S, Levy N, Yu J, Ip V, Mueller A, Banner-Goodspeed V, O'Gara B (2019) Virtual reality as an adjunct to anesthesia in the operating room. Trials 20(1):782. https://doi.org/10.1186/s13063-019-3922-2
Steele E, Grimmer K, Thomas B, Mulley B, Fulton I, Hoffman H (2003) Virtual reality as a pediatric pain modulation technique: a case study. Cyberpsychol Behav 6(6):633–638. https://doi.org/10.1089/109493103322725405
Esumi R, Yokochi A, Shimaoka M, Kawamoto E (2020) Virtual reality as a non-pharmacologic analgesic for fasciotomy wound infections in acute compartment syndrome: a case report. J Med Case Rep 14(1):1–7. https://doi.org/10.1186/S13256-020-02370-4/FIGURES/3
Huang MY, Scharf S, Chan PY (2020) Effects of immersive virtual reality therapy on intravenous patient-controlled sedation during orthopaedic surgery under regional anesthesia: a randomized controlled trial. PloS one 15(2):e0229320. https://doi.org/10.1371/journal.pone.0229320
Haisley KR, Straw OJ, Müller DT et al (2020) Feasibility of implementing a virtual reality program as an adjuvant tool for peri-operative pain control; results of a randomized controlled trial in minimally invasive foregut surgery. Complement Ther Med 49:102356. https://doi.org/10.1016/J.CTIM.2020.102356
Moon JY, Shin J, Chung J, Ji SH, Ro S, Kim WH (2018) Virtual reality distraction during endoscopic urologic surgery under spinal anesthesia: a randomized controlled trial. J Clin Med 8(1):2. https://doi.org/10.3390/jcm8010002
Eijlers R, Dierckx B, Staals LM et al (2019) Virtual reality exposure before elective day care surgery to reduce anxiety and pain in children: a randomised controlled trial. Eur J Anaesthesiol 36(10):728–737. https://doi.org/10.1097/EJA.0000000000001059
de Cacau LAP, Oliveira GU, Maynard LG et al (2013) The use of the virtual reality as intervention tool in the postoperative of cardiac surgery. Rev Bras Cir Cardiovasc 28(2):281–289. https://doi.org/10.5935/1678-9741.20130039
Noben L, Goossens SMTA, Truijens SEM, van Berckel MMG, Perquin CW, Slooter GD, van Rooijen SJ (2019) A virtual reality video to improve information provision and reduce anxiety before cesarean delivery: randomized controlled trial. JMIR Ment Health 6(12):e15872. https://doi.org/10.2196/15872
Jung MJ, Libaw JS, Ma K, Whitlock EL, Feiner JR, Sinskey JL (2021) Pediatric distraction on induction of anesthesia with virtual reality and perioperative anxiolysis: a randomized controlled trial. Anesth Analg 132(3):798–806. https://doi.org/10.1213/ANE.0000000000005004
Mosso-Vázquez JL, Gao K, Wiederhold BK, Wiederhold MD (2014) Virtual reality for pain management in cardiac surgery. Cyberpsychol Behav Soc Netw 17(6):371–378. https://doi.org/10.1089/CYBER.2014.0198
Ryu JH, Park SJ, Park JW et al (2017) Randomized clinical trial of immersive virtual reality tour of the operating theatre in children before anaesthesia. Br J Surg 104(12):1628–1633. https://doi.org/10.1002/bjs.10684
Chan JJI, Yeam CT, Kee HM, Tan CW, Sultana R, Sia ATH, Sng BL (2020) The use of pre-operative virtual reality to reduce anxiety in women undergoing gynecological surgeries: a prospective cohort study. BMC Anesthesiol 20(1):261. https://doi.org/10.1186/s12871-020-01177-6
Khanduja V, Lawrence JE, Audenaert E (2016) Testing the validity and utility of a virtual reality hip arthroscopy simulator. J Hip Preserv Surg 3(suppl_1):1–52. https://doi.org/10.1093/jhps/hnw030.001
Khanduja V, Lawrence JE, Audenaert E (2017) Testing the construct validity of a virtual reality hip arthroscopy simulator. Arthrosc - J Arthrosc Relat Surg 33(3):566–571. https://doi.org/10.1016/j.arthro.2016.09.028
Aggarwal R, Tully A, Grantcharov T et al (2006) Virtual reality simulation training can improve technical skills during laparoscopic salpingectomy for ectopic pregnancy. BJOG 113(12):1382–1387. https://doi.org/10.1111/J.1471-0528.2006.01148.X
Hooper J, Tsiridis E, Feng JE et al (2019) Virtual reality simulation facilitates resident training in total hip arthroplasty: a randomized controlled trial. J Arthroplasty 34(10):2278–2283. https://doi.org/10.1016/J.ARTH.2019.04.002
Larsen CR, Soerensen JL, Grantcharov TP et al (2009) Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ 338(7705):1253. https://doi.org/10.1136/BMJ.B1802
Teodoro-Vite S, Pérez-Lomelí JS, Domínguez-Velasco CF, Hernández-Valencia AF, Capurso-García MA, Padilla-Castañeda MA (2021) A high-fidelity hybrid virtual reality simulator of aneurysm clipping repair with brain sylvian fissure exploration for vascular neurosurgery training. Simul Healthc 16(4):285–294. https://doi.org/10.1097/SIH.0000000000000489
Logishetty K, Rudran B, Cobb JP (2019) Virtual reality training improves trainee performance in total hip arthroplasty: a randomized controlled trial. Bone Joint J 101-B(12):1585–1592. https://doi.org/10.1302/0301-620X.101B12.BJJ-2019-0643.R1
Kowalewski KF, Garrow CR, Proctor T et al (2018) LapTrain: multi-modality training curriculum for laparoscopic cholecystectomy-results of a randomized controlled trial. Surg Endosc 32(9):3830–3838. https://doi.org/10.1007/S00464-018-6110-7
Mccannel CA, Reed DC, Goldman DR (2013) Ophthalmic surgery simulator training improves resident performance of capsulorhexis in the operating room. Ophthalmology 120(12):2456–2461. https://doi.org/10.1016/J.OPHTHA.2013.05.003
Schulz GB, Grimm T, Kretschmer A, Stief CG, Jokisch F, Karl A (2020) Benefits and limitations of transurethral resection of the prostate training with a novel virtual reality simulator. Simul Healthc 15(1):14–20. https://doi.org/10.1097/SIH.0000000000000396
Gawęcki W, Węgrzyniak M, Mickiewicz P, Gawłowska MB, Talar M, Wierzbicka M (2020) The impact of virtual reality training on the quality of real antromastoidectomy performance. J Clin Med 9(10):1–11. https://doi.org/10.3390/JCM9103197
Mariani A, Pellegrini E, Enayati N, Kazanzides P, Vidotto M, De Momi E (2018) Design and evaluation of a performance-based adaptive curriculum for robotic surgical training: a pilot study. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf 2018:2162–2165. https://doi.org/10.1109/EMBC.2018.8512728
Cecil J, Gupta A, Pirela-Cruz M (2018) An advanced simulator for orthopedic surgical training. Int J Comput Assist Radiol Surg 13(2):305–319. https://doi.org/10.1007/S11548-017-1688-0
Nagayo Y, Saito T, Oyama H (2021) A novel suture training system for open surgery replicating procedures performed by experts using augmented reality. J Med Syst 45(5):60. https://doi.org/10.1007/s10916-021-01735-6
Kailavasan M, Berridge C, Athanasiadis G et al (2020) Design, implementation, and evaluation of a novel curriculum to teach transurethral resection of the prostate (TURP): a 3-year experience of urology simulation bootcamp course. World J Urol 38(11):2899–2906. https://doi.org/10.1007/S00345-020-03104-3
Beyer-Berjot L, Pucher P, Patel V et al (2017) Colorectal surgery and enhanced recovery: impact of a simulation-based care pathway training curriculum. J Visc Surg 154(5):313–320. https://doi.org/10.1016/J.JVISCSURG.2017.02.003
Botden SMBI, Buzink SN, Schijven MP, Jakimowicz JJ (2008) ProMIS augmented reality training of laparoscopic procedures face validity. Simul Healthc 3(2):97–102. https://doi.org/10.1097/SIH.0b013e3181659e91
Kroft J, Ordon M, Po L, Zwingerman N, Lee JY, Pittini R (2015) Can surgical “warm-up” with instructor feedback improve operative performance of surgical trainees? J Minim Invasive Gynecol 22(6S):S17–S18. https://doi.org/10.1016/J.JMIG.2015.08.057
Chugh AJ, Pace JR, Singer J et al (2017) Use of a surgical rehearsal platform and improvement in aneurysm clipping measures: results of a prospective, randomized trial. J Neurosurg 126(3):838–844. https://doi.org/10.3171/2016.1.JNS152576
Lendvay TS, Brand TC, White L et al (2013) Virtual reality robotic surgery warm-up improves task performance in a dry laboratory environment: a prospective randomized controlled study. J Am Coll Surg 216(6):1181–1192. https://doi.org/10.1016/J.JAMCOLLSURG.2013.02.012
Coelho G, Defino HLA (2018) The role of mixed reality simulation for surgical training in spine: phase 1 validation. Spine (Phila Pa 1976) 43(22):1609–1616. https://doi.org/10.1097/BRS.0000000000002856
Zwimpfer TA, Lacher D, Fellmann-Fischer B, Mueller M (2020) A laparoscopic study investigating 3D vs 2D imaging systems using a pelvitrainer model with experts, non-experts, and students. BMC Surg 20(1):276. https://doi.org/10.1186/s12893-020-00892-8
Gabrielli ME, Saun TJ, Jung, JJ, Grantcharov TP (2020) Assessment of 3-dimensional vs 2-dimensional imaging and technical performance using a multiport intraoperative data capture and analytic system for patients undergoing laparoscopic roux-en-Y gastric bypass surgery. JAMA network open 3(1):e1920084. https://doi.org/10.1001/jamanetworkopen.2019.20084
Patankar SB, Padasalagi GR (2017) Three-dimensional versus two-dimensional laparoscopy in urology: a randomized study. Indian J Urol 33(3):226. https://doi.org/10.4103/IJU.IJU_418_16
Bartlett JD, Lawrence JE, Stewart ME, Nakano N, Khanduja V (2018) Does virtual reality simulation have a role in training trauma and orthopaedic surgeons? Bone Joint J 100-B(5):559–565. https://doi.org/10.1302/0301-620X.100B5.BJJ-2017-1439
Bartlett JD, Lawrence JE, Yan M et al (2020) The learning curves of a validated virtual reality hip arthroscopy simulator. Arch Orthop Trauma Surg 140(6):761–767. https://doi.org/10.1007/s00402-020-03352-3
Logishetty K, Gofton WT, Rudran B, Beaulé PE, Cobb JP (2020) Fully immersive virtual reality for total hip arthroplasty: objective measurement of skills and transfer of visuospatial performance after a competency-based simulation curriculum. J Bone Joint Surg 102(6):e27. https://doi.org/10.2106/JBJS.19.00629
Xin B, Huang X, Wan W et al (2020) The efficacy of immersive virtual reality surgical simulator training for pedicle screw placement: a randomized double-blind controlled trial. Int Orthop 44(5):927–934. https://doi.org/10.1007/S00264-020-04488-Y
Blumstein G, Zukotynski B, Cevallos N et al (2020) Randomized trial of a virtual reality tool to teach surgical technique for tibial shaft fracture intramedullary nailing. J Surg Educ 77(4):969–977. https://doi.org/10.1016/J.JSURG.2020.01.002
Lohre R, Bois AJ, Athwal GS, Goel DP, Canadian Shoulder and Elbow Society (CSES) (2020) Improved complex skill acquisition by immersive virtual reality training: a randomized controlled trial. J Bone Joint Surg 102(6):e26
Orland MD, Patetta MJ, Wieser M, Kayupov E, Gonzalez MH (2020) Does virtual reality improve procedural completion and accuracy in an intramedullary tibial nail procedure? A randomized control trial. Clin Orthop Relat Res 478(9):2170–2177. https://doi.org/10.1097/CORR.0000000000001362
Bing EG, Brown ML, Cuevas A, Sullivan R, Parham GP (2021) User experience with low-cost virtual reality cancer surgery simulation in an african setting. JCO Glob Oncol 7(7):435–442. https://doi.org/10.1200/GO.20.00510
Marcus HJ, Pratt P, Hughes-Hallett A et al (2015) Comparative effectiveness and safety of image guidance systems in surgery: a preclinical randomised study. Lancet (London England) 385(Suppl 1):S64. https://doi.org/10.1016/S0140-6736(15)60379-8
Singla R, Edgcumbe P, Pratt P, Nguan C, Rohling R (2017) Intra-operative ultrasound-based augmented reality guidance for laparoscopic surgery. Healthc Technol Lett 4(5):204. https://doi.org/10.1049/HTL.2017.0063
Ma L, Zhao Z, Chen F, Zhang B, Fu L, Liao H (2017) Augmented reality surgical navigation with ultrasound-assisted registration for pedicle screw placement: a pilot study. Int J Comput Assist Radiol Surg 12(12):2205–2215. https://doi.org/10.1007/S11548-017-1652-Z
Zhu M, Liu F, Chai G, Pan JJ, Jiang, T, Lin, L, Xin Y, Zhang Y, Li Q (2017) A novel augmented reality system for displaying inferior alveolar nerve bundles in maxillofacial surgery. Sci Rep 7:42365. https://doi.org/10.1038/srep42365
Porpiglia F, Checcucci E, Amparore D, et al. (2021) Percutaneous kidney puncture with three-dimensional mixed-reality hologram guidance: from preoperative planning to intraoperative navigation. Eur Urol. Published online 2021. https://doi.org/10.1016/J.EURURO.2021.10.023
Gustafsson A, Pedersen P, Rømer TB, Viberg B, Palm H, Konge L (2019) Hip-fracture osteosynthesis training: exploring learning curves and setting proficiency standards. Acta Orthop 90(4):348–353. https://doi.org/10.1080/17453674.2019.1607111
Mohan A, Wara UU, Arshad Shaikh MT, Rahman RM, Zaidi ZA (2021) Telesurgery and robotics: an improved and efficient era. Cureus 13(3). https://doi.org/10.7759/CUREUS.14124
Author information
Authors and Affiliations
Contributions
V. Lu and J. Zhang performed full-text screening, data collection, and data analysis, and wrote the manuscript. V. Khanduja conceptualised the study and edited previous versions of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is a systematic review. The research ethics committee of the Cambridge University Hospitals NHS Foundation Trust has confirmed that no ethical approval is required.
Consent to participate
Not applicable.
Consent to publication
All authors have reviewed the final version and have consented to publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
James Zhang and Victor Lu contributed equally to this work and are co-first authors.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Lu, V. & Khanduja, V. The impact of extended reality on surgery: a scoping review. International Orthopaedics (SICOT) 47, 611–621 (2023). https://doi.org/10.1007/s00264-022-05663-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-022-05663-z