Abstract
Purpose
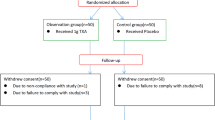
This prospective, stratified, randomized, single-blind, placebo-controlled multicentre study investigated the safety and effectiveness of reducing blood loss and preventing venous thromboembolism (VTE) during posterior lumbar interbody fusion (PLIF) in patients with stenosis or spondylolisthesis using the combination of tranexamic acid (TXA) and rivaroxaban.
Methods
The Autar score was evaluated in patients after admission. Patients with an Autar score ≤ 10 were randomized to group A or B. Group A was the placebo-controlled group. Patients in group B were treated with 1 g TXA via intravenous injection and 1 g TXA for external use. Patients with an Autar score > 10 were randomized to group C or D. Patients in group C were treated with 10-mg rivaroxaban qd for 35 days after surgery. Patients in group D received the same treatment as those in group B intra-operatively and as those in group C post-operatively.
Results
A total of 599 patients from eight hospitals participated in this clinical trial. The total blood loss, intra-operative blood loss, and drainage volume were reduced by the administration of TXA (group A vs group B, P < 0.01; group C vs group D, P < 0.01), and the blood transfusion rate was also decreased (group A vs group B, P < 0.01; group C vs group D, P < 0.01). There were no significant differences (P > 0.05) in the VTE incidence rates among group A and group B. In patients with high-risk thrombosis, the number of patients with VTE was only three and seven after the application of rivaroxaban. Epidural haematoma was not discovered in any patients in our trial.
Conclusion
The combined application of tranexamic acid and rivaroxaban significantly reduced the amount of blood loss and the transfusion rate during PLIF surgery and avoided an increase in the probability of thrombosis and the occurrence of epidural haematoma.
Trial registration number and date of registration
ChiCTR-1800016430
2018-06-01

Similar content being viewed by others
Data availability
We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality. The datasets generated during the current study are available in the Figshare repository https://figshare.com/s/b4128bd83127f2056e96.
References
Hu SS (2004) Blood loss in adult spinal surgery. Eur Spine J 13(Suppl 1):S3–S5
Szpalski M, Gunzburg R, Sztern B (2004) An overview of blood-sparing techniques used in spine surgery during the perioperative period. Eur Spine J 13(Suppl 1):S18–S27
Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F (2005) Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 102(4):727–732
Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB (1999) An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am 81(1):2–10
Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S (2001) Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg (Br) 83(5):702–705
Perel P, Clayton T, Altman DG et al (2014) Red blood cell transfusion and mortality in trauma patients: risk-stratified analysis of an observational study. PLoS Med 11(6):e1001664
Yang B, Li H, Wang D, He X, Zhang C, Yang P (2013) Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PLoS One 8(2):e55436
Wang Q, Liu J, Fan R et al (2013) Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J 22(9):2035–2038
Peters A, Verma K, Slobodyanyuk K et al (2015) Antifibrinolytics reduce blood loss in adult spinal deformity surgery: a prospective, randomized controlled trial. Spine (Phila Pa 1976) 40(8):E443–E449
Raksakietisak M, Sathitkarnmanee B, Srisaen P et al (2015) Two doses of tranexamic acid reduce blood transfusion in complex spine surgery: a prospective randomized study. Spine (Phila Pa 1976) 40(24):E1257–E1263
Shi H, Ou Y, Jiang D, Quan Z, Zhao Z, Zhu Y (2017) Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: a randomized trial. Medicine (Baltimore) 96(1):e5718
Kvederas G, Porvaneckas N, Andrijauskas A et al (2013) A randomized double-blind clinical trial of tourniquet application strategies for total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21(12):2790–2799
Xie J, Lenke LG, Li T et al (2015) Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients undergoing spine correction surgery. Spine J 15(4):647–654
Liang J, Liu H, Huang X et al (2016) Using tranexamic acid soaked absorbable gelatin sponge following complex posterior lumbar spine surgery: a randomized control trial. Clin Neurol Neurosurg 147:110–114
Kim KT, Kim CK, Kim YC et al (2017) The effectiveness of low-dose and high-dose tranexamic acid in posterior lumbar interbody fusion: a double-blinded, placebo-controlled randomized study. Eur Spine J 26(11):2851–2857
Cheriyan T, Maier SP 2nd, Bianco K et al (2015) Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J 15(4):752–761
Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H (2011) Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976) 36(23):1913–1918
Griffiths NJ (1979) Factors affecting the fibrinolytic response to surgery. Ann R Coll Surg Engl 61(1):12–16
Mannucci PM (1998) Hemostatic drugs. N Engl J Med 339(4):245–253
Mannucci PM, Levi M (2007) Prevention and treatment of major blood loss. N Engl J Med 356(22):2301–2311
Henry DA, Carless PA, Moxey AJ et al (2007) Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 4:CD001886
Gill JB, Chin Y, Levin A, Feng D (2008) The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am 90(11):2399–2407
Nishihara S, Hamada M (2015) Does tranexamic acid alter the risk of thromboembolism after total hip arthroplasty in the absence of routine chemical thromboprophylaxis. Bone Joint J 97-B(4):458–462
Levy JH, Moore KT, Neal MD et al (2018) Rivaroxaban reversal with prothrombin complex concentrate or tranexamic acid in healthy volunteers. J Thromb Haemost 16(1):54–64
Du W, Zhao C, Wang J, Liu J, Shen B, Zheng Y (2015) Comparison of rivaroxaban and parnaparin for preventing venous thromboembolism after lumbar spine surgery. J Orthop Surg Res 10:78
Shaieb MD, Watson BN, Atkinson RE (1999) Bleeding complications with enoxaparin for deep venous thrombosis prophylaxis. J Arthroplast 14(4):432–438
Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S (2004) Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 126(3 Suppl):287S–310S
Ricket AL, Stewart DW, Wood RC et al (2016) Comparison of postoperative bleeding in total hip and knee arthroplasty patients receiving rivaroxaban or enoxaparin. Ann Pharmacother 50(4):270–275
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Ethics approval
This retrospective, stratified, randomized, single-blind, placebo-controlled multicentre study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University (no. 2018059) and was registered in the Chinese Clinical Trial Registry (ChiCTR-1800016430).
Consent to participate
All subjects were aware of the clinical trial and agreed to participate.
Consent for publication
All authors have approved the manuscript and agree with submission to International Orthopaedics.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Li, Y., Liu, D. et al. Combined use of tranexamic acid and rivaroxaban in posterior lumbar interbody fusion safely reduces blood loss and transfusion rates without increasing the risk of thrombosis—a prospective, stratified, randomized, controlled trial. International Orthopaedics (SICOT) 44, 2079–2087 (2020). https://doi.org/10.1007/s00264-020-04699-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04699-3