Abstract
Purpose
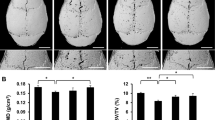
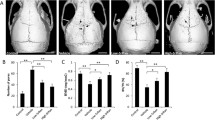
We examined the effects of triptolide on receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation and on titanium (Ti) particle-induced osteolysis.
Methods
To examine the effect of triptolide on osteoclast differentiation, bone marrow macrophages (BMMs) were treated with 100 ng/mL of RANKL and 30 ng/mL of macrophage-colony stimulating factor, or co-cultured with osteoblasts stimulated with 10 nM vitamin D3 and 1 μM prostaglandin E2 in the presence or absence of triptolide (2.8–14 nM). Osteoclast differentiation and activation were assessed using tartrate-resistant acid phosphatase staining, reverse transcriptase-polymerase chain reaction analysis to determine differentiation marker gene expression and pit formation assays. To examine the effect of triptolide on wear debris-induced osteolysis, titanium (Ti) particles were injected into the calvaria of ICR mice. Then, the mice were divided into three groups and were orally administered vehicle, or 16 or 32 μg/kg/day triptolide for ten days, followed by histomorphometric analysis.
Results
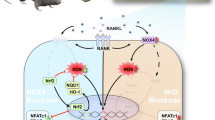
Triptolide suppressed RANKL-mediated osteoclast differentiation of BMMs in a dose-dependent manner. In a co-culture system, osteoblasts treated with triptolide could not induce osteoclast differentiation of BMMs, which was accompanied by down-regulation of RANKL and up-regulation of osteoprotegrin. Moreover, triptolide significantly inhibited bone resorption, and expression of the bone resorption marker genes. RANKL-induced activation of p38, ERK, and JNK was substantially inhibited by triptolide. Further, in a Ti-induced mouse calvarial erosion model, mice perorally administrated with triptolide showed significant attenuation of Ti-mediated osteolysis.
Conclusion
Our data indicated that triptolide had an anti-osteoclastic effect and significantly suppressed wear debris-induced osteolysis in mice.





Similar content being viewed by others
References
Wang J, Wang A, Zeng H, Liu L, Jiang W, Zhu Y, Xu Y (2012) Effect of triptolide on T-cell receptor beta variable gene mRNA expression in rats with collagen-induced arthritis. Anat Rec 295(6):922–927
Xue M, Jiang ZZ, Liu JP, Zhang LY, Wang T, Wang H, Liu L, Zhou ZX (2010) Comparative study on the anti-inflammatory and immune suppressive effect of Wilforlide A. Fitoterapia 81(8):1109–1112
Kupchan SM, Court WA, Dailey RG Jr, Gilmore CJ, Bryan RF (1972) Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc 94(20):7194–7195
Wong KF, Yuan Y, Luk JM (2012) Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin Exp Pharmacol Physiol 39(3):311–320
Wu Y, Cui J, Bao X, Chan S, Young DO, Liu D, Shen P (2006) Triptolide attenuates oxidative stress, NF-kappaB activation and multiple cytokine gene expression in murine peritoneal macrophage. Int J Mol Med 17(1):141–150
Qiu D, Kao PN (2003) Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D 4(1):1–18
Liu Q (2011) Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol 11(3):377–383
Xue X, Gong L, Qi X, Wu Y, Xing G, Yao J, Luan Y, Xiao Y, Li Y, Wu X, Chen M, Gu J, Ren J (2011) Knockout of hepatic P450 reductase aggravates triptolide-induced toxicity. Toxicol Lett 205(1):47–54
Li J, Jin J, Li M, Guan C, Wang W, Zhu S, Qiu Y, Huang M, Huang Z (2012) Role of Nrf2 in protection against triptolide-induced toxicity in rat kidney cells. Toxicol Lett 213(2):194–202
Ye X, Li W, Yan Y, Mao C, Cai R, Xu H, Yang X (2010) Effects of cytochrome P4503A inducer dexamethasone on the metabolism and toxicity of triptolide in rat. Toxicol Lett 192(2):212–220
Chen H, Chang X, Weng T, Zhao X, Gao Z, Yang Y, Xu H, Yang X (2004) A study of microemulsion systems for transdermal delivery of triptolide. J Control Release 98(3):427–436
Xu F, Shi X, Li S, Cui J, Lu Z, Jin Y, Lin Y, Pang J, Pan J (2010) Design, synthesis, and biological evaluation of novel water-soluble triptolide derivatives: Antineoplastic activity against imatinib-resistant CML cells bearing T315I mutant Bcr-Abl. Bioorg Med Chem 18(5):1806–1815
Fidler JM, Li K, Chung C, Wei K, Ross JA, Gao M, Rosen GD (2003) PG 490–88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol Cancer Ther 2(9):855–862
Aoyagi Y, Hitotsuyanagi Y, Hasuda T, Matsuyama S, Fukaya H, Takeya K, Aiyama R, Matsuzaki T, Hashimoto S (2008) Fluorination of triptolide and its analogues and their cytotoxicity. Bioorg Med Chem Lett 18(7):2459–2463
Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH (2012) Triptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep 29(4):457–475
Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK (2012) A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4(156):156ra139
Dumbleton JH, Manley MT, Edidin AA (2002) A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 17(5):649–661
Goldring SR, Schiller AL, Roelke M, Rourke CM, O’Neil DA, Harris WH (1983) The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am 65(5):575–584
Jin S, Park JY, Hong JM, Kim TH, Shin HI, Park EK, Kim SY (2011) Inhibitory effect of (-)-epigallocatechin gallate on titanium particle-induced TNF-alpha release and in vivo osteolysis. Exp Mol Med 43(7):411–418
Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL (1999) Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol 154(1):203–210
Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT (1995) Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. J Arthroplasty 10(4):498–506
Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP (2007) The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res 454:251–261
Ren W, Wu B, Peng X, Hua J, Hao HN, Wooley PH (2006) Implant wear induces inflammation, but not osteoclastic bone resorption, in RANK(−/−) mice. J Orthop Res 24(8):1575–1586
Ingham E, Fisher J (2005) The role of macrophages in osteolysis of total joint replacement. Biomaterials 26(11):1271–1286
Kwak HB, Lee BK, Oh J, Yeon JT, Choi SW, Cho HJ, Lee MS, Kim JJ, Bae JM, Kim SH, Kim HS (2010) Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone 46(3):724–731
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423(6937):337–342
Cappellen D, Luong-Nguyen NH, Bongiovanni S, Grenet O, Wanke C, Susa M (2002) Transcriptional program of mouse osteoclast differentiation governed by the macrophage colony-stimulating factor and the ligand for the receptor activator of NFkappa B. J Biol Chem 277(24):21971–21982
Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J (2001) Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J 20(6):1271–1280
Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K (2002) U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem 277(49):47366–47372
Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279(25):26475–26480
Nakashima T, Takayanagi H (2009) Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol 29(5):555–567
Zhao Q, Wang X, Liu Y, He A, Jia R (2010) NFATc1: functions in osteoclasts. Int J Biochem Cell Biol 42(5):576–579
Park JY, Bae MA, Cheon HG, Kim SS, Hong JM, Kim TH, Choi JY, Kim SH, Lim J, Choi CH, Shin HI, Kim SY, Park EK (2009) A novel PPARgamma agonist, KR62776, suppresses RANKL-induced osteoclast differentiation and activity by inhibiting MAP kinase pathways. Biochem Biophys Res Commun 378(3):645–649
Park EK, Lee YE, Choi JY, Oh SH, Shin HI, Kim KH, Kim SY, Kim S (2004) Cellular biocompatibility and stimulatory effects of calcium metaphosphate on osteoblastic differentiation of human bone marrow-derived stromal cells. Biomaterials 25(17):3403–3411
Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL (2003) c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest 111(5):749–758
Battaglino R, Kim D, Fu J, Vaage B, Fu XY, Stashenko P (2002) c-myc is required for osteoclast differentiation. J Bone Miner Res 17(5):763–773
Vukosavic S, Stefanis L, Jackson-Lewis V, Guegan C, Romero N, Chen C, Dubois-Dauphin M, Przedborski S (2000) Delaying caspase activation by Bcl-2: a clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 20(24):9119–9125
Choi YA, Lim J, Kim KM, Acharya B, Cho JY, Bae YC, Shin HI, Kim SY, Park EK (2010) Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. J Proteome Res 9(6):2946–2956
Wu Y, Wang Y, Zhong C, Li Y, Li X, Sun B (2003) The suppressive effect of triptolide on experimental autoimmune uveoretinitis by down-regulating Th1-type response. Int Immunopharmacol 3(10–11):1457–1465
Wang Y, Jia L, Wu CY (2008) Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. Scand J Immunol 68(4):383–390
Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N, Li JS (2010) Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol Immunol 47(15):2467–2474
Chen ZH, Qin WS, Zeng CH, Zheng CX, Hong YM, Lu YZ, Li LS, Liu ZH (2010) Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney Int 77(11):974–988
Liu C, Zhang Y, Kong X, Zhu L, Pang J, Xu Y, Chen W, Zhan H, Lu A, Lin N (2013) Triptolide prevents bone destruction in the collagen-induced arthritis model of rheumatoid arthritis by targeting RANKL/RANK/OPG signal pathway. Evid Based Complement Alternat Med 2013:626038
Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U (2012) Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol 74(3):424–436
Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, Crews CM (2007) Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci USA 104(11):4389–4394
McCallum C, Kwon S, Leavitt P, Shen DM, Liu W, Gurnett A (2007) Triptolide binds covalently to a 90 kDa nuclear protein. Role of epoxides in binding and activity. Immunobiology 212(7):549–556
Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y, Smeaton M, Demain AL, Miller PS, Kugel JF, Goodrich JA, Liu JO (2011) XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol 7(3):182–188
Hercus B, Saeed S, Revell PA (2002) Expression profile of T cell associated molecules in the interfacial tissue of aseptically loosened prosthetic joints. J Mater Sci Mater Med 13(12):1153–1156
Al-Saffar N, Khwaja HA, Kadoya Y, Revell PA (1996) Assessment of the role of GM-CSF in the cellular transformation and the development of erosive lesions around orthopaedic implants. Am J Clin Pathol 105(5):628–639
Cano E, Mahadevan LC (1995) Parallel signal processing among mammalian MAPKs. Trends Biochem Sci 20(3):117–122
Crotti TN, Smith MD, Findlay DM, Zreiqat H, Ahern MJ, Weedon H, Hatzinikolous G, Capone M, Holding C, Haynes DR (2004) Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials 25(4):565–573
Zhou B, Li X, Tang H, Miao Z, Feng H, Li Y (2011) Total synthesis of novel D-ring-modified triptolide analogues: structure-cytotoxic activity relationship studies on the D-ring of triptolide. Org Biomol Chem 9(9):3176–3179
Kupchan SM, Schubert RM (1974) Selective alkylation: a biomimetic reaction of the antileukemic triptolides? Science 185(4153):791–793
Zhang C, Peng F, Liu W, Wan J, Wan C, Xu H, Lam CW, Yang X (2014) Nanostructured lipid carriers as a novel oral delivery system for triptolide: induced changes in pharmacokinetics profile associated with reduced toxicity in male rats. Int J Nanomedicine 9:1049–1063
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MISP) (2008-0062282), and Kyungpook National University Research Fund, 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ju Ang Kim and Hye Jung Ihn contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 6
Cell viability of mouse BMM cells with triptolide. The effect of triptolide on BMM cells viability was determined using the MTT cytotoxicity assay. BMM cells were cultured in α-MEM containing 30 ng/mL M-CSF with or without 2.8, 7, or 14 nM of triptolide. (GIF 19 kb)
Rights and permissions
About this article
Cite this article
Kim, J.A., Ihn, H.J., Park, JY. et al. Inhibitory effects of triptolide on titanium particle-induced osteolysis and receptor activator of nuclear factor-κB ligand-mediated osteoclast differentiation. International Orthopaedics (SICOT) 39, 173–182 (2015). https://doi.org/10.1007/s00264-014-2596-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2596-3