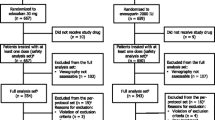
Abstract
Venous thromboembolism (VTE) is an important complication of major orthopaedic surgery of the lower limbs. Fondaparinux, a synthetic pentasaccharide and highly selective inhibitor of activated Factor Xa, is the first in a new class of antithrombotic agents. To determine the optimal dose in Japanese patients, double-blind, placebo-controlled, dose-ranging studies of fondaparinux were conducted in patients undergoing total knee replacement (TKR) or total hip replacement (THR) surgery. Patients were randomly assigned to receive a once-daily subcutaneous injection of fondaparinux (0.75, 1.5, 2.5, or 3.0 mg) or placebo in Study 1 (TKR) and Study 2 (THR). In Study 1, the incidence of VTE was 65.3% in the placebo group and was 34.2%, 21.3%, 16.2%, and 9.5% in the groups receiving 0.75, 1.5, 2.5, and 3.0 mg fondaparinux respectively. In Study 2, the incidence of VTE was 33.8% in the placebo group and was 24.2%, 4.6%, 7.4%, and 14.4% in the 0.75, 1.5, 2.5, and 3.0 mg fondaparinux groups respectively. Dose–response effects were observed in both studies; however, no statistically significant differences in major bleeding events were found among any groups. Fondaparinux proved to be a potent anticoagulant with a favourable benefit-to-risk ratio in the prevention of VTE in these study patients.
Résumé
Les complications thromboemboliques sont nombreuses dans la plupart des interventions de chirurgie orthopédique au niveau des membres inférieurs. Le fondaparinux (pentas saccharide synthétique) est un élément important parmi tous les agents anti-thrombotiques. De façon à déterminer la dose optimale de ce produit, une étude en double aveugle avec placebo a été conduite chez des patients devant bénéficier d’une prothèse totale du genou ou d’une prothèse totale de hanche. Les patients ont été randomisés de façon à recevoir une fois par jour une injection sous cutanée de fondaparinux (0.75, 1.5, 2.5, ou 3 mg) ou de placebo. L’incidence de la thrombose veineuse a été de 65.3% dans le groupe placebo et de 34.2%, 21.3%, 16.2% et 9.5% dans les groupes recevant respectivement 0.75, 1.5, 2.5 et 3 mg de fondaparinux, pour le groupe prothèse du genou. Pour le groupe prothèse de hanche l’incidence des complications thromboemboliques a été de 33.8% dans le groupe placebo et a été respectivement de 24.2%, 4.6%, 7.4% et 14.4% dans les groupes ayant reçu 0.75, 1.5, 2.5 et 3 mg de fondaparinux. Il n’y a pas de différence significatives en terme de saignement, dans chaque groupe. le fondaparinux est un anti-coagulant actif avec un bénéfice/risque important dans la prévention des thromboses veineuses et des accidents thromboemboliques dans cette étude de patients.

Similar content being viewed by others
References
Bauer KA, Eriksson BI, Lassen MR, Turpie AG (2001) The steering committee of the pentasaccharide in major knee surgery study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 345(18):1305–1310
Boneu B, Necciari J, Cariou R et al (1995) Pharmacokinetics and tolerance of the natural pentasaccharide (SR90107/ORG31540) with high affinity to antithrombin III in man. Thromb Haemost 74(6):1468–1473
Eriksson BI, Bauer KA, Lassen MR, Turpie AG (2001) The Steering committee of the Pentasaccharide in Hip-fracture surgery study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective hip-fracture surgery. N Engl J Med 345(18):1298–1304
Eriksson BI, Lassen MR, PENTasaccharide in HIp-FRActure Surgery Plus Investigators (2003) Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 163(11):1337–1342
Fujita S, Hirota S, Oda T, Kato Y, Tsukamoto Y, Fuji T (2000) Deep venous thrombosis after total hip or total knee arthroplasty in patients in Japan. Clin Orthop 375:168–174
Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, Wheeler HB (2001) Prevention of venous thromboembolism. Chest 119(1 Suppl):132S–175S
Geerts WH, Pineo GF, Heit JA et al (2004) Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic therapy. Chest 126(3 Suppl.):S338–S400
Herbert JM, Petitou M, Lormeau JC, Cariou R, Necciari J, Magnani HN et al (1997) SR 90107A/Org 31540, a novel anti-factor Xa antithrombic agent. Cardiovasc Drug Rev 15(1):1–26
Hirsh J, Hoak J (1996) Management of deep vein thrombosis and pulmonary embolism: a statement for healthcare professionals. From the council on thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 93(12):2212–2245
Lassen MR, Bauer KA, Eriksson BI, Turpie AG (2002) The European Pentasaccharide Hip Elective Surgery Study (EPHESUS) Steering Committee. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet 359(9319):1715–1720
Mann KG (1999) Biochemistry and physiology of blood coagulation. Thromb Haemost 82(2):165–74
Prescribing Information for Arixtra (fondaparinux). GlaxoSmithKline web site at http://us.gsk.com/products/assets/us_arixtra.pdf
Statistics and Information Department, Ministry of Health and Welfare: Patient Survey (1993) Tokyo, Japan (Japanese)
Statistics and Information Department, Ministry of Health and Welfare: Vital Statistics of Japan [Data compiled from 1964 to 1994] Tokyo, Japan. (Japanese)
Summary of Product Characteristics of fondaparinux. European Medicines Agency web site at http://www.emea.eu.int/humandocs/PDFs/EPAR/arixtra/H-403-PI-en.pdf#search=’arixtra’
Turpie AG, Gallus AS, Hoek JA (2001) Pentasaccharide Investigators. A synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacement. N Engl J Med 344(9):619–625
Turpie AG, Bauer KA, Eriksson BI, Lassen MR (2002) The PENTATHLON 2000 Study Steering Committee. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet 359(9319):1721–1726
Acknowledgements
These studies were supported by a grant from GlaxoSmithKline, Sanofi-synthelabo and NV Organon.
Members of Steering Committee
Takahiro Ochi (Chairman), National Sagamihara Hospital, Sagamihara
Takeo Matsuno, Orthopedic Surgery, Asahikawa Medical College, Asahikawa
Kozo Nakamura, Orthopedic Surgery, Tokyo University, School of Medicine, Tokyo
Tomihisa Koshino, International University of Health and Welfare, Tokyo
Hisashi Iwata, Center for Rheumatic Diseases and Artificial Joint, Nagoya Kyoritsu Hospital, Nagoya
Hideki Yoshikawa, Orthopedic Surgery, Osaka University, School of Medicine, Osaka
Takeshi Fuji, Orthopedic Surgery, Osaka Koseinenkin Hospital, Osaka
Toru Sato, Orthopedic Surgery, Okayama University, School of Medicine, Okayama
Sumiki Yamamoto, Centre for Rheumatic Diseases, Matsuyama Red Cross Hospital, Matsuyama
Takehiko Torisu, Orthopedic Surgery, Ohita Medical University, School of Medicine, Ohita
The members of the Central Independent Adjudication Committee for Efficacy (CIACE) of Study 1 & 2:
Satoru Fujita, Orthopaedic Surgery, Dai-ichi Hospital Medical Corporation of Showakai, Takarazuka
Hironobu Nakamura, Osaka University, Graduate School of Medicine Faculty of Medicine, Course of Advanced Medicine; Medical Robotics and Image Science, Osaka
Saki Nakata, Osaka University, Graduate School of Medicine Faculty of Medicine, Course of Advanced Medicine; Medical Robotics and Image Science, Osaka
Kenji Nakamura, Department of Radiology, Osaka City University Medical School, Osaka
The members of the central independent adjudication committee for safety (CIACS) of Study 1 & 2:
Hisaichi Fujii, Department of Transfusion and Cell Processing, Tokyo Women’s Medical University, School of Medicine, Tokyo
Ikuro Maruyama, Department of Laboratory Medicine; Faculty of Medicine, Kagoshima University, Kagoshima
Mitsuyoshi Nakashima, Hamamatsu University School of Medicine, Hamamatsu
Taisuke Tomatsu, Department of Rheumatology; Tokyo Women’s Medical University, School of Medicine, Tokyo
The principal investigators for Study 1 (TKR):
Yukiyoshi Oishi, Toyohashi Municipal Hospital, Toyohashi
Tatsunori Maeda, Asahikawa Medical College Hospital, Asahikawa
Hiroshi Tanaka, Yamaguchi University Hospital, Ube
Fujio Higuchi, Kurume University Medical Center, Kurume
Toshihisa Kanamono, Nagano Red Cross Hospital, Nagano
Takaharu Nabeshima, Toneyama National Hospital, Toyonaka
Masaaki Kakiuchi, Osaka Police Hospital, Osaka
Takeshi Fuji, Osaka Koseinenkin Hospital, Osaka
Yoshiaki Yanase, Kitano Hospital, Osaka
Takashi Soejima and Takahiro Ohkawa, Kurume University Hospital, Kurume
Hideto Machida, Kanto Rosai Hospital, Kawasaki
Hideji Kura, Sapporo Medical University Hospital, Sapporo
Hiromi Oda, The University of Tokyo Hospital, Tokyo
Masafumi Ishizuki, Tsuchiura Kyodo General Hospital, Tsuchiura
Makoto Kawakubo, Tokyo Dental College Ichikawa General Hospital, Ichikawa
Shoji Kumaki, Hokushin General Hospital, Nakano
Naoki Kodama, Gifu Prefectural Gero-Onsen Hospital, Gero
Masashi Kataoka, Oita Medical University Hospital, Hasama
Toshihiko Hara, Kyushu-Rosai Hospital, Kitakyushu
Shin-ichi Katsuo, Fukui General Hospital, Fukui
Naoto Mitsuki and Renzo Okamoto, Yokohama City University Medical Center, Yokohama
Tomoyuki Saito, Yokohama City University Hospital, Yokohama
Shigeru Harada, Tsukuba Rokujinkai Foundation Tsukuba Gakuen Hospital, Tsukuba
Masanori Shimode, Kanto Medical Center NTT EC, Tokyo
Yoshiki Okuda, Shakaihoken Kobe Central Hospital, Kobe
Hirofumi Kuroki, International Medical Center of Japan, Tokyo
Makoto Takasu, Aizu Chuo Hospital, Aizuwakamatsu
Tadashi Tanaka, Kimitsu Chuo Hospital, Kisarazu
Takahisa Yasoda and Naoto Mitsuki, Fujisawa Municipal Hospital, Fujisawa
Yukio Yoshida, Higashi Municipal Hospital of Nagoya, Nagoya
Kazuhiro Yamaguchi and Shinichiro Hara, Nagasaki Rosai Hospital, Sasebo
Toshihito Mori, National Sagamihara Hospital, Sagamihara
Kazuo Kaneko, Juntendo University Juntendo Izunagaoka Hospital, Izunagaoka
Sampei Nakata and Sumiki Yamamoto, Matsuyama Red Cross Hospital, Matsuyama
Toshikazu Tanaka, Tsukuba Memorial Hospital, Tsukuba
Taiki Kanno, Eniwa Hospital, Eniwa
Katsumi Chiba, Medical Corporation Fukushima Kouseikai Fukushima Daiichi Hospital, Fukushima
Shoichi Kushitani, Rinku General Medical Center, Izumisano
Masayoshi Ohga, Hiroshima Red Cross Hospital & Atomic-Bomb Survivors Hospital, Hiroshima
Toshiyuki Tsurumoto, Nagasaki University Hospital, Nagasaki
Etsuo Chosa, Miyazaki Medical College Hospital, Kiyotake
Chiaki Tanaka, Kyoto Municipal Hospital, Kyoto
Sen-eki Kobayashi, Shinshu University Hospital, Matsumoto
Shigeo Sano, Sanraku Hospital, Tokyo
Takashi Ohya, Obihiro Kosei Hospital, Obihiro
Kazunori Ohno, Teine Keijinkai Hospital, Sapporo
Katsuhiro Shimada, National Murayama Hospital, Musashi-murayama
Yoji Mikami, Japan Labour Health and Welfare Organization Yokohama Rosai Hospital, Yokohama
Akira Arakaki, Tomishiro Chuo Hospital, Tomigusuku
Yoshimitsu Hoshikawa, St Luke’s International Hospital, Tokyo
Shuji Okinaga, Tokyo Teishin Hospital, Tokyo
Syojiro Kato and Makoto Kurimura, Jinseisha Edogawa Hospital, Tokyo
Koji Suzuki, Hokkaido Orthopedic Memorial Hospital, Sapporo
Osamu Sugawara, Kitami Red Cross Hospital, Kitam
Kazuyoshi Hirose, Nagoya Kyoritsu Hospital, Nagoya-shi
Keiju Fujiwara, Osaka Prefectural General Hospital, Osaka
Takehiro Takebayashi, Sapporo Insurance General Hospital, Sapporo
Kazumasa Terada, National Kyushu Medical Center, Fukuoka
Hideya Kawamura, Kyushu Kousei Nenkin Hospital, Kitakyushu
The principal investigators for Study 2 (THR):
Yukiyoshi Oishi, Toyohashi Municipal Hospital, Toyohashi
Toru Sato, Okayama University Hospital, Okayama
Tadashi Teranishi, Asahikawa Medical College Hospital, Asahikawa
Hiroshi Tanaka, Yamaguchi University Hospital, Ube
Fujio Higuchi, Kurume University Medical Center, Kurume
Toshihisa Kanamono, Nagano Red Cross Hospital, Nagano
Takaharu Nabeshima, Toneyama National Hospital, Toyonaka
Masaaki Kakiuchi, Osaka Police Hospital, Osaka
Takeshi Fuji, Osaka Koseinenkin Hospital, Osaka
Yoshiaki Yanase, Kitano Hospital, Osaka
Takahiro Ohkawa and Masaru Kumagai, Kurume University Hospital, Kurume
Hideto Machida, Japan Labour Health and Welfare Organization Kanto Rosai Hospital, Kawasaki
Satoshi Nagoya, Sapporo Medical University Hospital, Sapporo
Masafumi Ishizuki, Tsuchiura Kyodo General Hospital, Tsuchiura
Masaaki Matsubara and Tetsuya Jinno, Tokyo Medical and Dental University Medical Hospital, Tokyo
Shoji Kumaki, Hokushin General Hospital, Nagano
Naoki Kodama, Gifu Prefectural Gero-Onsen Hospital, Gero
Toshihiko Hara, Kyushu-Rosai Hospital, Kitakyusyu
Shin-ichi Katsuo, Fukui General Hospital, Fukui
Kazuhiro Mizutani, Toho University Ohashi Hospital, Tokyo
Renzo Okamoto and Naoto Mitsuki, Yokohama City University Medical Center, Yokohama
Tomoyuki Saito, Yokohama City University Hospital, Yokohama
Shigeru Harada, Tsukuba Rokujinkai Foundation, Tsukuba Gakuen Hospital, Tsukuba
Masanori Shimode, Kanto Medical Center NTT EC, Tokyo
Yoshiki Okuda, Shakaihoken Kobe Central Hospital, Kobe
Hirofumi Kuroki, International Medical Center of Japan, Tokyo
Makoto Takasu, Aizu Chuo Hospital, Aizuwakamatsu
Tadashi Tanaka, Kimitsu Chuo Hospital, Kisarazu
Haruo Ito, Tokyo Koseinenkin Hospital, Tokyo
Naoto Mitsuki and Takahisa Yasoda, Fujisawa Municipal Hospital, Fujisawa
Yukio Yoshida, Higashi Municipal Hospital of Nagoya, Nagoya
Shinichiro Hara and Kazuhiro Yamaguchi, Nagasaki Rosai Hospital, Sasebo
Toshihito Mori, National Sagamihara Hospital, Fujisawa
Kazuo Kaneko, Juntendo University Juntendo Izunagaoka Hospital, Izunagaoka
Sumiki Yamamoto and Sampei Nakata, Matsuyama Red Cross Hospital, Matsuyama
Toshikazu Tanaka, Tsukuba Memorial Hospital, Tsukuba
Motoyuki Shundo, Eniwa Hospital, Eniwa
Katsumi Chiba, Medical Corporation Fukushima Kouseikai Fukushima Daiichi Hospital, Fukushima
Shoichi Kushitani, Rinku General Medical Center, Izumisano
Masayoshi Ohga, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital, Hiroshima
Hirosi Enomoto, Nagasaki University Hospital, Nagasaki
Hiroshi Usui, National Tokyo Medical Center, Tokyo
Etsuo Chosa, Miyazaki Medical College Hospital, Kiyotake
Chiaki Tanaka, Kyoto Municipal Hospital, Kyoto
Sen-eki Kobayashi, Shinshu University Hospital, Matsumoto
Shigeo Sano, Sanraku Hospital, Tokyo
Takashi Ohya, Obihiro Kosei Hospital, Obihiro
Katsuhiro Shimada, National Murayama Hospital, Musashi-murayama
Yoshimitsu Hoshikawa, St Luke’s International Hospital, Tokyo
Shuji Okinaga, Tokyo Teishin Hospital, Tokyo
Naoyuki Katayama, Hokkaido Orthopedic Memorial Hospital, Sapporo
Osamu Sugawara, Kitami Red Cross Hospital, Kitami
Kazuyoshi Hirose, Nagoya Kyoritsu Hospital, Nagoya
Keiju Fujiwara, Osaka Prefectural General Hospital, Osaka
Takehiro Takebayashi, Sapporo Insurance General Hospital, Sapporo
Hisaaki Miyahara, National Kyushu Medical Center, Fukuoka
Masanobu Saito, Osaka Minami National Hospital, Kawachinagano
Author information
Authors and Affiliations
Corresponding author
Additional information
For the Steering Committee of the Japan Fondaparinux Study in Arthroplasty.
Rights and permissions
About this article
Cite this article
Fuji, T., Fujita, S. & Ochi, T. Fondaparinux prevents venous thromboembolism after joint replacement surgery in Japanese patients. International Orthopaedics (SICO 32, 443–451 (2008). https://doi.org/10.1007/s00264-007-0360-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-007-0360-7