Abstract
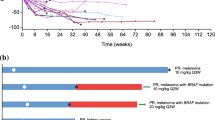
The class I IgG receptor (FcγRI or CD64 receptor), which is present on key cytotoxic effector cells, has been shown to initiate the destruction of tumor cells in vitro and has been hypothesized to play a role in the destruction of antibody-coated cells such as platelets in idiopathic thrombocytopenia purpura (ITP). This overview summarizes the clinical experience with CD64-directed immunotherapy in cancer patients with the bispecific antibodies MDX-447 [humanized Fab anti-CD64 × humanized Fab anti-(epidermal growth factor receptor, EGFR)] and MDX-H210 (humanized Fab anti-DC64 × Fab anti-HER2/neu), and with the anti-CD64 monoclonal antibody (mAB) MDX-33 (H22) in the modulation of monocyte CD64 in vivo. In an ongoing phase I/II open-label trial with progressive dose escalation (1–15 mg/m2), patients with treatment refractory EGFR-positive cancers (renal cell carcinoma (RCC), head and neck, bladder, ovarian, prostate cancer and skin cancer) are treated weekly with intravenous MDX-447, with and without granulocyte-colony-stimulating factor (G-CSF). MDX-447 has been found to be immunologically active at all doses, binding to circulating monocytes and neutrophils (when given with G-CSF), causing monocytopenia and stimulating increases in circulating plasma cytokines. MDX-447 is well tolerated, the primary toxicities being fever, chills, blood pressure lability, and pain/myalgias. Of 36 patients evaluable for response, 9 have experienced stable disease of 3–6 month’s duration. The optimal dose and the maximal tolerated dose (MTD) have yet to be defined; dose escalation continues to define better the dose, toxicity, and the potential therapeutic role of this bispecific antibody. Three MDX-H210 phase II trials are currently in progress, all using the intravenous dose of 15 mg/m2 given with granulocyte/macrophage (GM-CSF). These consist of one trial each in the treatment of RCC patients, patients with protrate cancer, and colorectal cancer patients, all of whom have failed standard therapy. At the time of writing, 11 patients have been treated in these phase II trials. Four patients have demonstrated antitumor effects. Patients demonstrating responses include 2 with RCC and 2 with prostate cancer. One RCC patient has had a 54% reduction in size of a hepatic metastatic lesion and the other has had a 49% decrease in the size of a lung metastasis with simultaneous clearing of other non-measurable lung lesions. Regarding the two patients with prostate cancer, one has had a 90% reduction in serum prostate-specific antigen (PSA; 118–11 ng/ml), which has persisted for several months; the other patient with prostate has had a 70% reduction of serum PSA (872 ng/ml to 208 ng/ml) within the first month of treatment. Both patients have also demonstrated symptomatic improvement. In a completed phase I and in ongoing phase I/II clinical trials, patients with treatment-refractory HER2/neu positive cancers (breast, ovarian, colorectal, prostate) have been treated with MDX-H210, which has been given alone and in conjunction with G-CSF, GM-CSF, and interferon γ (IFNγ). These trials have been open-label, progressive dose-escalation (0.35–135 mg/m2) studies in which single, and more often, multiple weekly doses have been administered. MDX-H210 has been well tolerated, with untoward effects being primarily mild-to-moderate flu-like symptoms. The MTD has not yet been defined. MDX-H210 is immunologically active, binding to circulating monocytes, causing monocytopenia, as well as stimulating increases in plasma cytokine levels. Furthermore, some patients have evidence of active antitumor immunity following treatment with MDX-210. Antitumor effects have been seen in response to MDX-H210 administration; these include 1 partial, 2 minor, and 1 mixed tumor response; 15 protocol-defined stable disease responses have occurred. In a completed phase I trial, MDX-33 was administered as a single intravenous dose to 17 normal subjects in order to assess its potential as an immunomodulator for the treatment of idiopathic thrombocytopenia purpura and other immune disorders. Doses of 1.5, 3.0, 5.0, and 7.5 mg/m2 were administered. The variables evaluated in response to MDX-33 were circulating monocyte and neutrophil counts, monocyte CD64-mediated phagocytosis, monocyte CD64 modulation, MDX-33 pharmocokinetics, and various safety paramenters. MDX-33 is well tolerated at doses of 5.0 mg/m2 or less, the primary toxicities being chills, low-grade fever, headache, and muscle aches. Persistent binding of MDX-33 to 80–99 % of circulating monocytes is seen for at least 6 days; down-modulation of monocyte CD64 occurs and also lasts more than 6 days. Monocyte CD64-mediated phagocytosis is significantly inhibited at all doses of MDX-33. At the 3.0 mg/m2 and 5.0-mg/m2 dose, phagocytosis is fully inhibited for at least 6 days, returning to baseline levels by 20 days after dosing. These results clearly demonstrate that immunomodulation of monocyte CD64 by the mAB MDX-33 can be accomplished with minimal clinical toxicity, and further indicate the potential of MDX-33 in the treatment of ITP and other auto-immune disorders. In conclusion, the results from completed and ongoing clinical trials with the CD64-directed bsAB MDX-447 and MDX-H210 demonstrate excellent tolerability in association with promising antitumor effects in tumors that have become refractory to all available therapies. Also promising are the results from the trial of the CD64-directed mAB, MDX-33, which show the ability to modulate monocyte CD64 in the clinical setting. Studies are currently being conducted to elucidate the full potential of these and other approaches using CD64-directed immunotherapy.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Accepted: 14 October 1997