Abstract
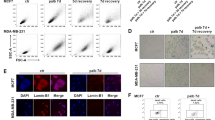
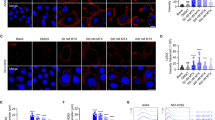
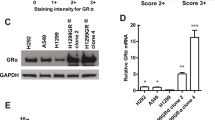
There is a need to improve response rates of immunotherapies in lung adenocarcinoma (AC). Extended (7–14 days) treatment of high glucocorticoid receptor (GR) expressing lung AC cells with dexamethasone (Dex) induces an irreversible senescence phenotype through chronic induction of p27. As the senescence-associated secretory phenotype (SASP) may have either tumor supporting or antitumor immunomodulatory effects, it was interest to examine the effects of Dex-induced senescence of lung AC cells on immune cells. Dex-induced senescence resulted in sustained production of CCL2, CCL4, CXCL1 and CXCL2, both in vitro and in vivo. After Dex withdrawal, secretion of these chemokines by the senescent cells attracted peripheral blood monocytes, T-cells, and NK cells. Following treatment with Dex-induced SASP protein(s), the peripheral blood lymphocytes exhibited higher cell count and tumor cytolytic activity along with enhanced Ki67 and perforin expression in T and NK cells. This cytolytic activity was partially attributed to NKG2D, which was upregulated in NK cells by SASP while its ligand MICA/B was upregulated in the senescent cells. Enhanced infiltrations of T and NK cells were observed in human lung AC xenografts in humanized NSG mice, following treatment with Dex. The findings substantiate the idea that induction of irreversible senescence in high-GR expressing subpopulations of lung AC tumors using Dex pretreatment enhances tumor immune infiltration and may subsequently improve the clinical outcome of current immunotherapies.






Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Reck M, Rodriguez-Abreu D, Robinson AG et al (2019) Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37:537–546. https://doi.org/10.1200/JCO.18.00149
Reck M, Remon J, Hellmann MD (2022) First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol 40:586–597. https://doi.org/10.1200/JCO.21.01497
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW (2007) Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445:656–660. https://doi.org/10.1038/nature05529
Lasry A, Ben-Neriah Y (2015) Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol 36:217–228. https://doi.org/10.1016/j.it.2015.02.009
McHugh CI, Thipparthi MR, Lawhorn-Crews JM et al (2018) Using radiolabeled 3’-Deoxy-3’-(18)F-fluorothymidine with PET to monitor the effect of dexamethasone on non-small cell lung cancer. J Nucl Med 59:1544–1550. https://doi.org/10.2967/jnumed.117.207258
Patki M, Gadgeel S, Huang Y, McFall T, Shields AF, Matherly LH, Bepler G, Ratnam M (2014) Glucocorticoid receptor status is a principal determinant of variability in the sensitivity of non-small-cell lung cancer cells to pemetrexed. J Thorac Oncol 9:519–526. https://doi.org/10.1097/JTO.0000000000000111
Patki M, McFall T, Rosati R et al (2018) Chronic p27(Kip1) induction by dexamethasone causes senescence phenotype and permanent cell cycle blockade in lung adenocarcinoma cells over-expressing glucocorticoid receptor. Sci Rep 8:16006. https://doi.org/10.1038/s41598-018-34475-8
Parajuli P, Anand R, Mandalaparty C et al (2016) Preferential expression of functional IL-17R in glioma stem cells: potential role in self-renewal. Oncotarget 7:6121–6135. https://doi.org/10.18632/oncotarget.6847
Paladugu M, Thakur A, Lum LG, Mittal S, Parajuli P (2013) Generation and immunologic functions of Th17 cells in malignant gliomas. Cancer Immunol Immunother 62:75–86. https://doi.org/10.1007/s00262-012-1312-7
Griffith JW, Sokol CL, Luster AD (2014) Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32:659–702. https://doi.org/10.1146/annurev-immunol-032713-120145
Robertson MJ (2002) Role of chemokines in the biology of natural killer cells. J Leukoc Biol 71:173–183
Rao SG, Jackson JG (2016) SASP: tumor suppressor or promoter? Yes. Trends Cancer 2:676–687. https://doi.org/10.1016/j.trecan.2016.10.001
Ruhland MK, Coussens LM, Stewart SA (2016) Senescence and cancer: an evolving inflammatory paradox. Biochim Biophys Acta 1865:14–22. https://doi.org/10.1016/j.bbcan.2015.10.001
Velarde MC, Demaria M, Campisi J (2013) Senescent cells and their secretory phenotype as targets for cancer therapy. Interdiscip Top Gerontol 38:17–27. https://doi.org/10.1159/000343572
Franchimont D, Galon J, Vacchio MS et al (2002) Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor alpha. J Immunol 168:2212–2218
Morgan DJ, Davis DM (2017) Distinct effects of dexamethasone on human natural killer cell responses dependent on cytokines. Front Immunol 8:432. https://doi.org/10.3389/fimmu.2017.00432
Perez SA, Mahaira LG, Demirtzoglou FJ et al (2005) A potential role for hydrocortisone in the positive regulation of IL-15-activated NK-cell proliferation and survival. Blood 106:158–166. https://doi.org/10.1182/blood-2004-08-3232
Moustaki A, Argyropoulos KV, Baxevanis CN, Papamichail M, Perez SA (2011) Effect of the simultaneous administration of glucocorticoids and IL-15 on human NK cell phenotype, proliferation and function. Cancer Immunol Immunother 60:1683–1695. https://doi.org/10.1007/s00262-011-1067-6
Li Y, He F, Liu S, Zhang Y, Li L, Wang B, Lan L, Pan Z (2021) Effect of pretreatment with dexamethasone on the efficacy and immune-related adverse events of immunotherapy in first-line treatment for advanced non-small cell lung cancer: a network meta-analysis of randomized control trials. Am J Clin Exp Immunol 10:93–102
Wabnitz GH, Michalke F, Stober C, Kirchgessner H, Jahraus B, van den Boomen DJ, Samstag Y (2011) L-plastin phosphorylation: a novel target for the immunosuppressive drug dexamethasone in primary human T cells. Eur J Immunol 41:3157–3169. https://doi.org/10.1002/eji.201041366
Waltzer WC, Bachvaroff RJ, Arnold A, Anaise D, Rapaport FT (1985) Immunological consequence of renal transplantation and immunosuppression: I—alterations in human natural killer-cell activity. J Clin Immunol 5:78–83
Hepburn B, Slade JD (1987) Effect of divided daily dose prednisone therapy on circulating T cell subsets. J Rheumatol 14:19–22
Kiuchi Z, Nishibori Y, Kutsuna S et al (2019) GLCCI1 is a novel protector against glucocorticoid-induced apoptosis in T cells. FASEB J 33:7387–7402. https://doi.org/10.1096/fj.201800344RR
Acknowledgements
Our institutional Microscopy, Imaging and Cytometry Resources (MICR) core facility were used for the flow cytometry studies. The Core is supported, in part, by NIH Center Grant P30 CA22453 to the Karmanos Cancer Institute, Wayne State University, and the Perinatology Research Branch of the National Institutes of Child Health and Development. We are thankful to Jeremy M. Kelm for thorough evaluation of the manuscript for language and grammar.
Funding
This study was partially supported by a donation to the Parajuli Lab from Marvin Klein Trust, Michigan.
Author information
Authors and Affiliations
Contributions
PP, NG and MR conceptualized the study, analyzed data and wrote the manuscript; RR performed in vitro and in vivo (SCID mice model) senescence induction studies; SD, LP and NG helped with the in vivo (NSG mice model) studies; HM helped with study design, data analysis and interpretation; REW, ZH, and AN performed all cell culture, migration and cytotoxicity experiments, prepared Figures and wrote Methods.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parajuli, P., Rosati, R., Mamdani, H. et al. Senescence-associated secretory proteins induced in lung adenocarcinoma by extended treatment with dexamethasone enhance migration and activation of lymphocytes. Cancer Immunol Immunother 72, 1273–1284 (2023). https://doi.org/10.1007/s00262-022-03332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03332-z