Abstract
Background
Inflammatory biomarkers in the peripheral blood have been established as predictors for immunotherapeutic efficacy in advanced non-small cell lung cancer (NSCLC). Whether they can also predict major pathological response (MPR) in neoadjuvant setting remains unclear.
Methods
In this multi-center retrospective study, 122 and 92 stage I-IIIB NSCLC patients from six hospitals who received neoadjuvant chemoimmunotherapy followed by surgery were included in the discovery and external validation cohort, respectively. Baseline and on-treatment neutrophil-to-lymphocyte ratio (NLR), derived NLR (dNLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR) and systemic immune-inflammation index (SII) were calculated and associated with MPR. Furthermore, resected tumor samples from 37 patients were collected for RNA-sequencing to investigate the immune-related tumor microenvironment.
Results
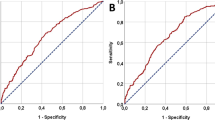
In both the discovery and validation cohorts, the on-treatment NLR, dNLR, PLR, and SII levels were significantly lower in the patients with MPR versus non-MPR. On-treatment SII remained an independent predictor of MPR in multivariate logistic regression analysis. The area under the curve (AUC) of on-treatment SII for predicting MPR was 0.75 (95%CI, 0.67–0.84) in the discovery cohort. Moreover, the predictive value was further improved by combining the on-treatment SII and radiological tumor regression data, demonstrating an AUC of 0.82 (95%CI, 0.74–0.90). The predictive accuracy was validated in the external cohort. Compared with the SII-high group, patients with SII-Low were associated with the activated B cell receptor signaling pathway and a higher intratumoral immune cell infiltration level.
Conclusions
On-treatment SII was independently associated with MPR in NSCLC patients receiving neoadjuvant chemoimmunotherapy. Further prospective studies are warranted.




Similar content being viewed by others
Data availability
The data that support the finding of our study are available on request from the corresponding author.
Change history
20 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00262-022-03294-2
References
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 378(22):2078–2092
Forde PM, Chaft JE, Smith KN et al (2018) Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 378(21):1976–1986
Shu CA, Gainor JF, Awad MM et al (2020) Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21(6):786–795
Provencio M, Nadal E, Insa A et al (2020) Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21(11):1413–1422
Jiang L, Huang J, Jiang S et al (2021) The surgical perspective in neoadjuvant immunotherapy for resectable non-small cell lung cancer. Cancer Immunol Immunother 70(8):2313–2321
Wu J, Hou L, E H, et al. Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer. 2022;165:115–23.
Hellmann MD, Chaft JE, William WN Jr et al (2014) Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 15(1):e42-50
Gao S, Li N, Gao S et al (2020) Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 15(5):816–826
Jomrich G, Paireder M, Kristo I et al (2021) High Systemic Immune-Inflammation Index is an Adverse Prognostic Factor for Patients With Gastroesophageal Adenocarcinoma. Ann Surg 273(3):532–541
Valero C, Lee M, Hoen D et al (2021) Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun 12(1):729
Fornarini G, Rebuzzi SE, Banna GL et al (2021) Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: an analysis of the Italian SAUL cohort. ESMO Open 6(3):100118
Nenclares P, Gunn L, Soliman H, et al. On-treatment immune prognostic score for patients with relapsed and/or metastatic head and neck squamous cell carcinoma treated with immunotherapy. J Immunother Cancer. 2021;9(6).
Fukui T, Okuma Y, Nakahara Y et al (2019) Activity of Nivolumab and Utility of Neutrophil-to-Lymphocyte Ratio as a Predictive Biomarker for Advanced Non-Small-Cell Lung Cancer: A Prospective Observational Study. Clin Lung Cancer 20(3):208–14.e2
Shang J, Han X, Zha H et al (2021) Systemic Immune-Inflammation Index and Changes of Neutrophil-Lymphocyte Ratio as Prognostic Biomarkers for Patients With Pancreatic Cancer Treated With Immune Checkpoint Blockade. Front Oncol 11:585271
Capone M, Giannarelli D, Mallardo D et al (2018) Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 6(1):74
Goldstraw P, Chansky K, Crowley J et al (2016) The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 11(1):39–51
Travis WD, Dacic S, Wistuba I et al (2020) IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 15(5):709–740
Ritchie ME, Phipson B, Wu D et al (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43(7):e47
Barbie DA, Tamayo P, Boehm JS et al (2009) Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462(7269):108–112
Charoentong P, Finotello F, Angelova M et al (2017) Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep 18(1):248–262
Pavlou M, Ambler G, Seaman SR et al (2015) How to develop a more accurate risk prediction model when there are few events. BMJ 351:h3868
Rothschild SI, Zippelius A, Eboulet EI et al (2021) SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J Clin Oncol 39(26):2872–2880
Zhang J, Ji Z, Caushi JX et al (2020) Compartmental Analysis of T-cell Clonal Dynamics as a Function of Pathologic Response to Neoadjuvant PD-1 Blockade in Resectable Non-Small Cell Lung Cancer. Clin Cancer Res 26(6):1327–1337
Laza-Briviesca R, Cruz-Bermúdez A, Nadal E et al (2021) Blood biomarkers associated to complete pathological response on NSCLC patients treated with neoadjuvant chemoimmunotherapy included in NADIM clinical trial. Clin Transl Med 11(7):e491
Casarrubios M, Cruz-Bermúdez A, Nadal E et al (2021) Pretreatment Tissue TCR Repertoire Evenness Is Associated with Complete Pathologic Response in Patients with NSCLC Receiving Neoadjuvant Chemoimmunotherapy. Clin Cancer Res 27(21):5878–5890
Russo A, Russano M, Franchina T et al (2020) Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Outcomes with Nivolumab in Pretreated Non-Small Cell Lung Cancer (NSCLC): A Large Retrospective Multicenter Study. Adv Ther 37(3):1145–1155
Bagley SJ, Kothari S, Aggarwal C et al (2017) Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106:1–7
Möller M, Turzer S, Schütte W et al (2020) Blood Immune Cell Biomarkers in Patient With Lung Cancer Undergoing Treatment With Checkpoint Blockade. J Immunother 43(2):57–66
Li M, Spakowicz D, Burkart J et al (2019) Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol 145(10):2541–2546
Park CK, Oh HJ, Kim MS et al (2021) Comprehensive analysis of blood-based biomarkers for predicting immunotherapy benefits in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res 10(5):2103–2117
Suh KJ, Kim SH, Kim YJ et al (2018) Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 67(3):459–470
Sekine K, Kanda S, Goto Y et al (2018) Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer 124:179–188
Chen Y, Wen S, Xia J et al (2021) Association of Dynamic Changes in Peripheral Blood Indexes With Response to PD-1 Inhibitor-Based Combination Therapy and Survival Among Patients With Advanced Non-Small Cell Lung Cancer. Front Immunol 12:672271
Bauckneht M, Genova C, Rossi G, et al. The Role of the Immune Metabolic Prognostic Index in Patients with Non-Small Cell Lung Cancer (NSCLC) in Radiological Progression during Treatment with Nivolumab. Cancers (Basel). 2021;13(13).
Chowell D, Yoo SK, Valero C, et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat Biotechnol. 2021. https://doi.org/10.1038/s41587-021-01070-8
Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17(12):e542–e551
Hawinkels LJ, Zuidwijk K, Verspaget HW et al (2008) VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer 44(13):1904–1913
Schmied L, Höglund P, Meinke S (2021) Platelet-Mediated Protection of Cancer Cells From Immune Surveillance - Possible Implications for Cancer Immunotherapy. Front Immunol 12:640578
Alessi JV, Ricciuti B, Alden SL, et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J Immunother Cancer. 2021;9(11).
Helmink BA, Reddy SM, Gao J et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577(7791):549–555
Acknowledgements
We ae grateful to all the members in the multidisciplinary team for their efforts.
Funding
This study was supported by National Key Research and Development Program of China (2021YFC2500904 and 2021YFC2500905), Shanghai Municipal Health Commission (202040322, 201940192, 2019SY072), Shanghai Hospital Development Center (SHDC22021217), National Natural Science Foundation of China (No. 81972176), and the Science Foundation of Shanghai (No.18ZR1435100).
Author information
Authors and Affiliations
Contributions
The study conducts and design: CL, JW, LJ, and LZ, QL, and CC; data acquisition: CL, JW, JH, YT, XL, LX, LH, MY, MM, CS, HZ, and HC; data analysis and interpretation: CL, JW, LJ, YZ, HE, PG, YS, and DX; drafting the manuscript or revising it: all authors; final approval of the manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethics Approval
This study was approved by the Institutional Review Board of Shanghai Pulmonary Hospital (IRB number: L21-224). The IRB waived the patient’s informed consent as this was a non-interventional study using routinely collected data.
Consent for publication
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Only Dong Xie is listed as the corresponding author. Authors Qingquan Luo (luoqingquan@hotmail.com) and Chang Chen (chenthoracic@163.com) should be listed as well.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Wu, J., Jiang, L. et al. The predictive value of inflammatory biomarkers for major pathological response in non-small cell lung cancer patients receiving neoadjuvant chemoimmunotherapy and its association with the immune-related tumor microenvironment: a multi-center study. Cancer Immunol Immunother 72, 783–794 (2023). https://doi.org/10.1007/s00262-022-03262-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03262-w