Abstract
Wilms’ tumor 1 (WT1) is a promising tumor-associated antigen for cancer immunotherapy. We developed an oral protein vaccine platform composed of WT1-anchored, genetically engineered Bifidobacterium longum (B. longum) and conducted an in vivo study in mice to examine its anticancer activity. Mice were orally treated with phosphate-buffered saline, wild-type B. longum105-A, B. longum 2012 displaying only galacto-N-biose/lacto-N-biose I-binding protein (GLBP), and WT1 protein- and GLBP-expressing B. longum 420. Tumor size reduced significantly in the B. longum 420 group than in the B. longum 105-A and 2012 groups (P < 0.00 l each), indicating B. longum 420’s antitumor activity via WT1-specific immune responses. CD8+ T cells played a major role in the antitumor activity of B. longum 420. The proportion of CD103+CD11b+CD11c+ dendritic cells (DCs) increased in the Peyer’s patches (PPs) from mice in the B. longum 420 group, indicating the definite activation of DCs. In the PPs, the number and proportion of CD8+ T cells capable of producing interferon-gamma were significantly greater in the B. longum 420 group than in the B. longum 2012 group (P < 0.05 or < 0.01). The production of WT1-specific IgG antibody was significantly higher in the B. longum 420 group than in the 2012 group (P < 0.05). The B. longum 420 group showed the most intense intratumoral infiltration of CD4+ and CD8+ T cells primed by activated DCs in the PPs of mice in the B. longum 420 group. Our findings provide insights into a novel, intestinal bacterium-based, cancer immunotherapy through intestinal immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bifidobacterium longum (B. longum) strains have been shown to stimulate CD4+ T cells and promote Th1 responses [1]. Probiotic bacteria, when taken into dendritic cells (DCs), serve to CD8+ T cell priming [1,2,3,4]. These players are the constituents of CD4+ T cell help in cancer immunotherapy. We developed an oral vaccine platform using B. longum as an antigen-delivering vehicle to the intestinal immune system and generated a Wilms’ tumor 1 (WT1) oral anticancer vaccine that expresses WT1 protein—a tumor-associated antigen (TAA) [5]. WT1, which is overexpressed in most adult and pediatric patients with acute leukemia, chronic myelocyte leukemia, and myelodysplastic syndrome [6], is considered most promising among 75 TAAs as a target for cancer vaccines and/or T cell adaptive immunotherapy [7].
The plasmid carrying galacto-N-biose/lacto-N-biose I-binding protein (GLBP)-murine WT1 fusion gene” was introduced into a wild-type B. longum 105-A by electroporation to produce B. longum 420, i.e., a product of pharmaceutical devisal consisting of WT1-anchored, genetically engineered B. longum to augment the efficiency of priming of T cells by professional antigen-presenting cells, dendritic cells (DCs). GLBP was used to express WT1 protein on the B. longum surface.
The objectives of the present study were (1) to identify which immunocompetent cells in the intestinal lymphatic system (Peyer’s patches [PPs], lamina propria, and mesenteric lymph nodes [MLNs]) are activated by the vaccine and (2) to examine how the vaccine is involved in the cellular mechanisms of intestinal immunity that result in exerting its antitumor activity on a target tumor via the systemic circulation.
Materials and methods
Animals and study design
C57BL/6 J (H-2Db) mice (5–6 weeks old; female; 3–17 animals per study group) were purchased from CLEA Japan, Inc. Body weight of tumor inoculated animals was measured at specified time points. All animal studies were conducted in compliance with regulatory and academic regulations (including humane endpoints) and were approved by Osaka University Animal Experiment Committee.
Cells
C1498, a murine leukemia cell line of C57BL/6 origin without expression of WT1 protein, was obtained from ATCC (Rockville, MD, USA). Murine WT1-expressing C1498 (C1498-mWT1) was generated by transducing C1498 cells with CMV promotor-driven murine WT1 17AA(+) KTS(+) isoform full-length cDNA that had been inserted into the pcDNA3.1(+) mammalian expression vector (Invitrogen, Tokyo, Japan).
WT1 oral anticancer vaccines using wild-type B. longum 105-A and genetically engineered B. longum
Genetically engineered B. longum 2012 displays only GLBP on the bacterial cell surface, while B. longum 420 displays a partial murine WT1 protein (117–419 amino acid residues) via GLBP. A plasmid carrying the GLBP-coding gene was introduced into wild-type B. longum 105-A by electroporation to generate B. longum 2012, while another plasmid carrying the GLBP-murine WT1 fusion gene was used to generate B. longum 420 [5]. B. longum 105-A and B. longum 2012 were used as controls. B. longum 105-A was cultured anaerobically in the Gifu Anaerobic Medium (GAM) broth (Nissui, Tokyo, Japan) at 37 °C. B. longum 2012 and B. longum 420 were cultured anaerobically in the GAM broth with 15 µg/ml spectinomycin (Sigma-Aldrich, St. Louis, MO) at 37 °C. All these three strains were washed with phosphate-buffered saline (PBS) and were then suspended to obtain the final cell density of 2 × 109 colony-forming units (CFUs)/100 µl.
In vivo leukemia cell inoculation
2 × 105 C1498-mWT1 cells in 50 µl of PBS were inoculated subcutaneously to the right dorsal side of C57BL/6 J mice on day 0. Tumor growth was monitored every 2 days. Tumor volume was calculated using the following formula: (longest diameter) × (shortest diameter) × (height) × 1/2.
In vivo CD8+ T cell depletion study
To deplete CD8+ T cells, the anti-CD8 monoclonal antibody (mAb) was prepared using the hybridoma clones in vivo in BALB/c nude mice. C57BL/6 J mice were intraperitoneally injected with 500 mg of the anti-CD8 mAb for 3 consecutive days (days -8, -7, and -6) and day -3 before day 0 when leukemia cells were subcutaneously inoculated and every 3 days since day 0 [8,9,10]. On day -2, CD8+ T cell depletion was verified by the flow cytometry (FCM) analysis of peripheral blood mononuclear cells (PBMCs) collected from the tail vein, and the analysis was conducted every 7 days since day 0 to confirm the sustained depletion of CD8+ T cells. For the analysis, T cells were stained with the anti-CD8-Alexa Fluor 647 (KT15, MBL, Nagoya, Japan), anti-CD3-FITC (17A2, eBioscience, San Diego, CA), anti-CD4-V500, (RPA-T4, BD Biosciences, Franklin Lakes, NJ), and anti-CD8a-V450 (53-6.7, BD Biosciences) mAbs. Tumor growth was monitored every 2 days.
Immunohistochemical staining
Paraffin-embedded tumor tissues were used in the immunohistochemical staining of CD4+ and CD8+ T cells. A microtome (Leica, Wetzlar, DEU) was used to prepare the 4-µm-thick tissue sections before deparaffinization and rehydration. Antigen epitopes were retrieved by autoclaving at 121 °C for 20 min in 10 mM sodium citrate buffer (pH 6.0). The sections were incubated overnight at 4 °C with the rabbit anti-CD4 (Bioss, Boston, MA) or anti-CD8 (Abcam, Cambridge, UK) antibody that was diluted 1:100. Subsequently, the sections were incubated for 60 min at room temperature with the secondary antibody HRP-conjugated anti-rabbit IgG (H + L) (Promega, Madison, WI). Positive signals were visualized by using 3,3’-diaminobenzidine (Sigma-Aldrich) for 3 min before the sections were counterstained with hematoxylin for 5 min. As negative controls, the sections were stained in parallel with isotype control, the rabbit monoclonal IgG antibody (Abcam). CD4- or CD8-positive cells were counted as described previously [11].
WT1 tetramer assay
PBMCs were collected from the tail vein of 5 mice in each study group to determine the frequency of CD8+ T cells recognizing the WT1 epitope WT1126 (a.a.126-134). The multicolor staining of PBMCs was conducted with a PE-conjugated WT1126-134 tetramer (MBL). Cells were incubated with anti-CD8-Alexa Fluor 647 (MBL) and anti-CD3-FITC (eBioscience), and then with 7-AAD (BD Biosciences). Fluorescence was detected and analyzed using the FACSCanto™ II (BD Biosciences) flow cytometer and the FlowJo™ software (BD Biosciences).
Chromium 51 (51Cr) release cytotoxicity assay
C1498-mWT1 tumor-bearing mice received PBS, B. longum 105-A, B. longum 2012, or B. longum 420 on days 1 to 10; animals were killed on day 11 after leukemia cell inoculation. The cytotoxic activity of WT1-specific cytotoxic T cells (CTLs) was evaluated in the 51Cr release assay as described previously [12, 13]. An H-2Db-binding peptide, WT1126-134 peptide (a.a.126-134; Scrum, Tokyo, Japan), was dissolved in PBS; splenocytes were collected and stimulated with 5 μg/ml WT1126-134 peptide and were then cultured in 10% fetal bovine serum (FBS; Thermo Fischer Scientific, Waltham, MA), 45% RPMI 1640 (Sigma-Aldrich), 45% AIM-V (Gibco, Waltham, MA), 1 × nonessential amino acid (Gibco), 25 ng/ml 2-mercaptoethanol (FUJIFILM Wako Pure Chemical, Osaka, Japan), and 50 IU/ml penicillin-50 μg/ml streptomycin (FUJIFILM Wako Pure Chemical). Two and 4 days later, recombinant interleukin-2 (rIL-2; Kyowa Pharmaceutical Industry, Osaka, Japan) was added to the culture at a concentration of 20 IU/ml. After 6-day culture, the 51Cr release cytotoxicity assay was conducted to examine the WT1-specific cytotoxic activity of splenocytes on target RMAS cells with or without the addition of WT1126-134 peptide. The percentage of specific lysis (% specific lysis [count per minute of experimental release—count per minute of spontaneous release]/[count per minute of maximal release—count per minute of spontaneous release] × 100) was calculated at various effector/target (E/T) ratios for WT1126-134-pulsed and -unpulsed RMAS cells that were lysed by splenocytes from mice in the PBS, B. longum 105-A, B. longum 2012, and B. longum 420 groups.
Enzyme-linked immune sorbent assay (ELISA) for anti-WT1 IgG antibody titers
WT1-specific IgG antibodies were assayed by ELISA. Briefly, 96-well plates were filled with WT1 protein (MyBioSource, San Diego, CA) and were then incubated at 4 °C. After incubation, the plates were washed with tris-buffered saline with 0.05% Tween 20 (TBST; Sigma) and were then blocked with gelatin-containing TBST for 2 h at room temperature. C57BL/6 J mice were treated with 100 µl of PBS, B. longum 105-A, B. longum 2012, or B. longum 420 (2 × 109 CFUs/100 µl of PBS) every day. On day 28, mice plasma was collected and diluted to 1:1000 with TBST, and 100 µl of diluted plasma were added to each well for incubation at 4 °C. All samples were measured in duplicate wells. Absorbance at a wavelength of 550 nm was recorded using the MTP-310 microplate reader (Hitachi, Tokyo, Japan). ELISA endpoint titers were defined as “the mean absorbance in two wells for a single sample containing serum”—“the absorbance in a well for a single sample that was free of serum.” 6F-H2, an anti-WT1 antibody (Millipore, Temecula, CA), was used as positive control, and buffer was used as negative control.
Multicolor fluorescence in situ hybridization (FISH) for the detection of B. longum
C57BL/6 J mice were orally given 100 µl of PBS or B. longum 420 (2 × 109 CFUs/100 µl of PBS) every day for 10 days. On day 11, the small intestine was removed and then fixed with the Carnoy’s solution before paraffin embedding. The rRNA sequence specific to B. longum (Blon1004) (AGCCGTATCTCTACGACCGT) was labeled with Cy3 at the 5’ end (Sigma-Aldrich) [14]. The Cy5-labeled oligonucleotide probe Eub338—which is complementary to a region of 16S rRNA highly conserved in the domain Bacteria [15]—was also used to detect the presence of B. longum 420 in the lumen of the small intestine and the PPs. The 100-µl solution of hybridization containing 1 µg each of these probes was poured onto each section. The sections were mounted with the VECTASHIELD® Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). MojoSort™ Mouse CD11c Nanobeads (Biolegend, San Diego, CA) were used to positively sort CD11c+ DCs in the PPs from mice that had been orally given B. longum 420 for 15 days. The resulting DCs were separated into two aliquots for May-Giemsa staining and FISH.
Intracellular cytokine staining
C57BL/6 J mice were orally given 100 µl of PBS, B. longum 2012, or B. longum 420 (2 × 109 CFUs/100 µl of PBS) every day. On day 29, animals (n = 8/group) were killed to remove the small intestine including the PPs, MLNs, and colon. The expressions of interferon (IFN)-γ+CD4+ and IFN-γ+CD8+ T cells in the PPs, MLNs, small intestine, and colon were analyzed with the Cytofix/Cytoperm™ Plus Kit with GolgiStop™ (BD Biosciences) according to the manufacturer’s instructions. In brief, the cells were incubated with 50 ng/ml PMA (phorbol 12-myristate 13-acetate; Sigma-Aldrich), 5 μM calcium ionophore A23187 (Sigma-Aldrich), and GolgiStop™ in RPMI 1640. Surface staining was conducted with the anti-CD8-APC-Cy7 antibody (53-6.7, BD Biosciences) and the anti-CD4-Percp-Cy5.5 antibody (CK1.5, BD Biosciences), and intracellular cytokine staining was conducted with the FITC-conjugated anti-IFN-γ antibody (XMG1.2, BD Biosciences) [16]. Samples were analyzed using the FACSCanto™ II flow cytometer and the FlowJo™ software.
Flow cytometry of the PPs, MLNs, and spleen
C57BL/6 J mice were orally given 100 µl of PBS, B. longum 105-A, B. longum 2012, or B. longum 420 (2 × 109 CFUs/100 µl of PBS) every day for 10 days. On day 11, animals (n = 8/group) were killed to remove the small intestine including the PPs, MLNs, and spleen. Cells in the PPs, MLNs, and spleen were incubated with the following antibodies: anti-CD45-APC-Cy7 (30-F11, BD Biosciences), anti-CD11c-PE-Cy7(HL3, BD Biosciences), anti-major histocompatibility (MHC) class I-A/I-E (MHC II)-BV421 (M5/114.15.2, BD Biosciences), anti-CD103-APC (M290, BD Biosciences), anti-CD11b-BV510 (M1/70, BD Biosciences), and 7-AAD (BD Biosciences), and anti-CD86-FITC (GL-1, Biolegend). Samples were analyzed using the FACSCanto™ II flow cytometer and the FlowJo™ software. MHC class II+CD11c+ cells were gated from 7-AAD−CD45+ cells, followed by the analysis of DC activation based on the measured levels of CD86 and by the subset analysis of DCs through the levels of CD103 and CD11b [17, 18].
Statistical analysis
Comparisons among multiple groups were conducted by one-way analysis of variance, followed by Student’s t test. A P value of < 0.05 was considered statistically significant. All statistical analyses were conducted with JMP® version 14.1 (SAS Institute Inc., Cary, NC) and the SAS software version 9.4 (SAS Institute Inc.).
Results
In vivo antitumor activity of B. longum
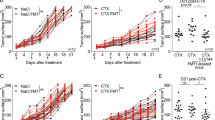
We examined the anticancer activity of the following materials: controls that did not express WT1 protein—PBS, wild-type B. longum 105-A, and B. longum 2012 displaying only GLBP; and WT1 protein- and GLBP-anchored B. longum 420 on the bacterial cell surface. Subsequent to day 10 when successful tumor engraftment was verified, mice received PBS, B. longum 105-A, B. longum 2012, and B. longum 420. Body weights of any animals in the study groups did not decrease during the study period. The average volume of the C1498-mWT1 tumor mass was significantly smaller in the B. longum 420 group than in the B. longum 2012 group on days 17, 19, and 21 after leukemia cell inoculation (P < 0.01, P < 0.001, and P < 0.001, respectively; Fig. 1a); hence, the antitumor activity of B. longum 420 was shown to be caused by WT1-specific immune responses. Furthermore, tumor size on day 17 after leukemia cell inoculation was significantly smaller in the B. longum 105-A and 2012 groups than in the PBS group (P < 0.05 each; Fig. 1a).
Antitumor activity of study materials, intratumoral infiltration and number of T cells, % specific lysis of target RMAS cells by splenocytes, frequency of WT1-specific CTLs, and anti-WT1 IgG antibody titers. a Average tumor volume changes after leukemia cell inoculation (n = 17 each). Scale bars represent standard error. *: P < 0.05, **: P < 0.01, ***: P < 0.001. Two-sided Student’s t test, except for day 21 that was analyzed by Dunnett test. Successful tumor engraftment was verified on day 10 when the oral administration of study materials was started. b The upper diagram showing the study schedule in mice, including the frequency of FCM. The lower diagram showing average tumor volume changes after leukemia cell inoculation with or without the depletion of CD8+ T cells, followed by the oral administration of PBS and B. longum 420 starting on day 12. Scale bars represent standard error. Two-sided Student’s t test. c After tumor inoculation on day 0, oral administration of study materials was conducted on days 1 to 28. Animals were killed to remove the tumor. Immunohistochemical stains of TILs. Images are representative of 2 and 3 experiments for CD4+ and CD8+ T cells, respectively. Scale bar, 50 µm. Arrowheads, tumor-infiltrating CD4+ and CD8+ T cells. d Number of CD4+ T cells in the study groups (n = 5 each). Red lines denote the mean values. e Number of CD8+ T cells in the study groups (n = 5 each). Red lines denote the mean values. f Successful tumor engraftment was verified on day 10 when the oral administration of study materials was started. On day 18 after leukemia cell inoculation into animals of the study group (n = 5 each), the tetramer assay was conducted to determine the frequency (%) of WT1-specific CTLs whose T cell receptor recognize a complex consisting of H-2Db and the WT1 epitope WT1126 (a.a.126-134)—RMFPNAPYL. g Representative diagrams of flow cytometry plotting WT1 tetramer-positive CD8+ T cells in mice treated with PBS, B. longum 105-A, B. longum 2012, and B. longum 420. h C1498-mWT1 cells were subcutaneously inoculated on day 0. Oral administration was conducted on days 1 to 10. On day 11, % specific lysis of WT1126-134 peptide-pulsed target RMAS cells (a TAP-deficient subline of RMA—Rauscher leukemia virus-induced lymphoma cell line of C57BL/6 origin) by splenocytes was determined. Solid diamond (WT1126-134 peptide-pulsed RMAS cells), open diamond (WT1126-134 peptide-unpulsed RMAS cells). Mice treated with PBS, B. longum 105-A, B. longum 2012, and B. longum 420. Representative data in triplicate are shown. i Oral administration was conducted on days 1 to 28. The anti-WT1 IgG antibody titer, expresses as absorbance at a wavelength of 550 nm, was significantly greater in the B. longum 420 (n = 7) group than in the PBS (n = 11), B. longum 105-A (n = 9), and B. longum 2012 (n = 8). PBS, phosphate-buffered saline; B. longum, Bifidobacterium longum; FCM, flow cytometry; i.p., intraperitoneal; PB, peripheral blood; p.o. per os; CTLs, cytotoxic T cells; PBMCs, peripheral blood mononuclear cells; E/T, effector target ratio
In vivo CD8+ T cell-dependent antitumor activity of B. longum 420
On day 25 after leukemia cell inoculation, tumor size was significantly smaller in the B. longum 420 group than in the PBS group (P < 0.05). However, tumor size became nearly equal between the anti-CD8 antibody + B. longum 420 group and the PBS group (Fig. 1b). Namely, the administration of anti-CD8 antibody counterbalanced the antitumor activity of the B. longum 420 group, indicating that CD8+ T cells are the main effector of the B. longum 420 group.
Infiltrations of CD4+ and CD8+ T cells in the C1498-mWT1 mass
The infiltrations of CD4+ and CD8+ T cells in the C1498-mWT1 mass (CD4+ or CD8+ tumor-infiltrating lymphocytes [TILs]) are shown in Fig. 1c, and the numbers of CD4+ and CD8+ TILs in Fig. 1d and Fig. 1e, respectively. The number of CD4+ T cells was significantly greater in the B. longum 420 group than in the PBS and B. longum 105-A groups (P < 0.01 and P < 0.05; Fig. 1d). The number of CD8+ T cells was significantly greater in the B. longum 420 group than in the PBS, B. longum 105-A, and B. longum 2012 groups (P < 0.001, P < 0.01, and P < 0.01; Fig. 1e). Namely, mice treated with B. longum 420 showed the most intense intratumor infiltration of CD4+- and CD8+- T cells.
Frequency of WT1-specific CTLs and production of anti-WT1 protein IgG antibody
In a tetramer assay that detects WT1-specific CTLs which recognize WT1126-134 peptide (i.e., the H-2Db-restricted epitope of WT1 protein), we then determined WT1-specific CTL frequency in CD8+ T cells collected from PBMCs. The frequency of WT1-specific CTLs significantly greater in the B. longum 420 group than in the PBS, B. longum 105-A, and B. longum 2012 groups (P < 0.01 each; Fig. 1f); the representative data thereof are shown in Fig. 1g. The cytotoxic activity (as expressed in % specific lysis) of splenocytes on WT1126-134-pulsed RMAS cells was shown in the B. longum 420 group but not in the other study 3 groups (Fig. 1h).
CD4+ T cell help is considered to activate and/or expand CTLs. We assessed the activation of WT1-specific CD4+ T cells by examining the production of the anti-WT1 IgG antibody because CD4+ T cells stimulate the class-switch of IgM to IgG [19]. At week 4, absorbance at a wavelength of 550 nm reflecting the titers was significantly greater in the B. longum 420 group than in other study groups (P < 0.05 each; Fig. 1i).
FISH assay of B. longum in the PPs
4′6-Diamidino-2-phenylindole (DAPI) and probes—Blon1004 (Cy3-labeled) and Eub338 (Cy5-labeled)—were used to conduct the multicolor FISH of the small intestine from mice in an attempt to determine the uptake thereof into the intestine including the PPs as manifested in blue, green, and red, respectively (Fig. 2). B. longum 420 was found not only in the lumen of the small intestine (Fig. 2a) but also in DCs from the PPs (Fig. 2b) in the FISH images.
Localization of bacterial rRNA in the small intestine including Peyer’s patches. Mice were treated orally with PBS and B. longum 420 on days 1 to 10. a On day 11, animals were killed to remove the small intestine. FISH of the rRNA of B. longum 420 taken in the small intestinal lumen and walls of mice treated with PBS or B. longum 420 by using DAPI (blue), as well as probes—Blon1004 (green) and Eub338 (red). Representative data in triplicate are shown. Scale bar, 10 µm. Arrows, the rRNA of B. longum 420 taken in the small intestinal lumen. b On day 11, animals were killed to conduct FISH of the rRNA of B. longum 420 taken in by DCs in the PPs by using DAPI (blue), as well as probes—Blon1004 (green) and Eub338 (red). Representative data in triplicate are shown. Scale bar, 10 µm. Arrows, bacterial rRNA taken in by DCs. Magnification (bar: 20 µm) to indicate the better presentation of cytoplasmic fluorescence rRNA, ribosomal ribonucleic acid; FISH, fluorescence in situ hybridization; DAPI, 4′6-diamidino-2-phenylindol; PBS, phosphate-buffered saline; PPs, Peyer’s patches; DCs, dendritic cells
Next, we examined the mechanisms of intestinal immunity generating the antitumor activity of B. longum. Tumor-nonbearing mice were orally given PBS, B. longum 2012, or B. longum 420 for 4 weeks. On day 29, cells were collected from the PPs, MLNs, small intestine, and colon, followed by the stimulation thereof with PMA and calcium ionophore to examine the production of IFN-γ (Fig. 3a and b, respectively). The numbers and proportions of IFN-γ+CD4+ T cells in the PPs were significantly greater in the B. longum 420 group than in the B. longum 2012 group (P < 0.05 or < 0.01; Fig. 3a, b), which indicated the greater functionality of CD4+ T cells in the PPs for mice in the B. longum 420 group than for mice in the B. longum 2012 group.
Immunization with Bifidobacterium. a Oral administration of study materials was conducted on days 1 to 28. On day 29, the number and proportion of IFN-γ+CD4+ T cells in the PPs, MLNs, small intestine, and colon were determined (n = 8/group). Scale bars represent SE. b The number and proportion of IFN-γ+CD8+ T cells in the PPs, MLNs, small intestine, and colon were determined (n = 8/group). Scale bars represent SE IFN, interferon; PBS, phosphate-buffered saline; PPs, Peyer’s patches; MLNs, mesenteric lymph nodes
The numbers and proportions of IFN-γ+CD4+ and IFN-γ+CD8+ T cells in the MLNs were significantly greater in the B. longum 2012 and 420 groups than in the PBS group, excepting the proportion of IFN-γ+CD8+ T cells (P < 0.05 or < 0.01; Fig. 3b), which indicated the greater antitumor functionality of CD4+ and CD8+ T cells in the MLNs for mice in the B. longum 2012 and 420 groups than for mice in the PBS group.
Activation of DCs by intestinal immunity
The proportion of CD86+ DCs among MHC class II+CD11c+ DCs in the PPs was greater for B. longum 105-A (9.3%) and 2012 (10.8%) than for PBS (5.1%) and was markedly greater for B. longum 420 (24.5%) than for other 2 bacterial strains (Fig. 4a). Furthermore, the proportion of CD103+CD11b+CD11c+ DCs was greater for B. longum 105-A (16.0%) and 2012 (9.10%) than for PBS (7.73%) (Fig. 4b) and was markedly greater for B. longum 420 (31.2%) than for other 2 bacterial strains. These CD103+ DCs, which captured bacterial rRNA, were found more abundant in the PPs from mice in the B. longum 420 group, implying the good recognition and presentation of a TAA—WT1—by DCs in the entry of the lymphocytic network leading to systemic circulation. Activated DCs in the PPs, which were stained by May-Giemsa staining, are shown in Fig. 4c (i), and inactive ones in Fig. 4c (ii). The presence of Blon1004-labeled B. longum 420 taken in by CD103+CD11c+ DCs in the PPs (Fig. 4c (iii)) corroborates the flowcytometric data of Fig. 4b in contrast to CD103−CD11c+ DCs that did not take in B. longum 420. Figure 4c (iv) indicates the nonuptake of B. longum 420 by CD103− DCs resulting in a failure to activate DCs.
Staining of dendric cells in the Peyer’s patches, MLN and spleen. Representative data in triplicate are shown. Oral administration of B. longum 420 was conducted on days 1 to 10, and mice were killed on day 11. a Flow cytometry indicating the proportion of CD86+ DCs in the PPs, MLNs, and spleen. b Flow cytometry indicating the proportion of CD11b+CD11c+CD103+ DCs in the PPs, MLNs, and spleen. On day 15 of oral administration, two aliquots of DCs were collected from the PPs of mice that were treated with B. longum 420. One aliquot of CD11c+ DCs sorted from the PPs was used to conduct May-Giemsa staining: 4 representative, activated DCs c-i) and 2 inactivated DCs c-ii). Another aliquot of representative, activated DCs c-iii) and inactive DCs c-iv)—which were stained with the anti-CD103-APC antibody (M290, BD Biosciences)—were deposited onto a slide according to the cytospin method before their hybridization to conduct FISH using DAPI (blue), CD103 (red), and Blon1004 (green). Representative data in triplicate are shown. Scale bar, 2 µm. DCs, dendritic cells; PP, Peyer’s patches; FISH, fluorescence in situ hybridization; DAPI, 4′6-diamidino-2-phenylindol
Discussion
The present study elucidated immunological mechanisms by which this B. longum-based oral anticancer vaccine triggers intestinal immunity in mice, identified immunocompetent cells in the immune system—PPs and MLNs, and afforded the following findings: (1) CD8+ T cells played the major role in the cytotoxic activity of B. longum 420, with the significantly greater activity compared to B. longum 2012; (2) only B. longum 420 induced WT1-specific CTLs; (3) CD4+ T cell help was evidenced by the production of anti-WT1 IgG antibody and by the increased number and proportion of CD4+ T cells capable of producing IFN-γ; (4) B. longum 420 taken in by DCs in the PPs induced the generation of CD103+CD11b+CD11c+ DCs capable of efficiently presenting WT1 peptides on the cell surface to prime CD8+ T cells; and (5) the activation of DCs by B. longum strains and by the add-on effect of WT1 anchoring.
CD8+ T cells played the major role in the antitumor activity of B. longum 420 because the administration of the anti-CD8 antibody completely suppressed the activity to mice in the B. longum 420 group. CD8+ T cells significantly infiltrated in the tumor mass of mice in the B. longum 420 group compared to those in the B. longum 105-A and 2012 groups. The frequency of WT1-specific CTLs in PBMCs is significantly greater in the former group than in the latter groups. The antitumor activity of B. longum 420 is WT1-specific because the cytotoxic activity of CD8+ T cells on WT1 peptide-pulsed RMAS cells increased in the B. longum 420 group, implying that CD8+ T cells collected from mice in the B. longum 420 group recognize WT1 peptide and are capable of lysing WT1-expressing tumor cells. Namely, CD8+ T cells are the principal immunologic competent cells that exerted the antitumor activity of B. longum 420. Furthermore, the production of anti-WT1 IgG antibody in the B. longum 420 group infers the involvement of CD4+ T cell help.
B. longum DNA contains immunostimulatory motifs that trigger an innate immunity [20], and three B. longum strains examined in the present study activated DCs in the PPs and suggested the antigen-independent antitumor activity thereof based on innate immunity.
Of special note was the finding that B. longum 420 exerted antitumor activity despite the fact that the proportion of CD103+ DCs—which have conventionally been considered to reduce intestinal immune responses by stimulating regulatory T cells in the intestine [21]—was increased by B. longum 420. Our data suggest that WT1 peptides cross-presented by CD103+ DCs in the PPs to CD8+ T cells prime or reactive CTLs in the systemic circulation via the MLNs, leading to the exertion of the antitumor activity of B. longum 420, as inferred by a previous study [22].
Besides WT1-specific T cell immunity, we demonstrated that T cell immunity in the PPs and MLNs was enhanced by two B. longum-based oral vaccines. Subsequent to the stimulation of cells from the PPs and MLNs by PMA and calcium ionophore, the number and proportion of IFN-γ+CD4+ T cells in the PPs from mice were significantly greater in the B. longum 420 group than in the B. longum 2012 group, which indicated that CD4+ T cells in the PPs from mice were functionally more active in the B. longum 420 group than in the B. longum 2012 group. This finding is consistent with our demonstration that more DCs are activated in the PPs from mice in the B. longum 420 group than in the B. longum 2012 group, since activated DCs stimulated both WT1-specific and -nonspecific CD4+ T cells in the PPs. Furthermore, CD4+ T cells in the MLNs from mice were functionally more active in the B. longum 2012 and 420 groups than in the PBS group; however, the reason for this finding was unknown because the proportions of CD103+ DCs in the MLNs were equivalent between the B. longum 2012 and 420 groups. Oral vaccination with B. longum strains may stimulate T cells in the MLNs due to unelucidated mechanisms.
In association with the above-mentioned activation states of T cells in the PPs and MLNs, the B. longum 420 group showed the most intense intratumoral infiltration of CD4+ and CD8+ T cells presumably to due to (1) the nonspecific activation of DCs by Bifidobacterium itself and to (2) the priming of WT1-specific, cytokine-producing CD4+ and CD8+ T cells that are activated by DCs in the PPs which, in turn, further stimulates these DCs. The efficient activation of DCs and immunological priming of CD8+ T cells by B. longum 420 at the PPs and MLNs appear to have contributed to the exertion of the antitumor activity thereof. Further investigation is needed to confirm this speculation.
In contrast to a Lactococcus lactis oral vaccine expressing human papillomavirus type 16 E7 under development for exogenous viruses [23], our oral vaccine has the potential of clinical applicability to many solid and hematologic malignancies of endogenous origin. However, further research is required to investigate differences in the phenotypic features of CD103+ DCs with antitumor activity in the intestinal and systemic immune systems.
In conclusion, the present study indicates that this oral anticancer vaccine triggers intestinal immunity to exert the antitumor activity of WT1-specific CD8+ T cells possibly through CD4+ T cell help, and we speculate that unidentified components of the systemic immune system (e.g., the function of natural killer cells and macrophages, as well as phenotypes of CD4+ T cells) appear to be involved in the antitumor activity of the vaccine. This oral WT1 protein vaccine has clinical applicability because of its potential advantages: high safety, low-cost manufacturing, easy scale-up for massive production, and expectation for good adherence by cancer patients, especially pediatric patients.
Availability of data and material
All data are available in the manuscript on a reasonable request. Correspondence should be addressed to Yoshiko Hashii, MD, PhD (yhashii@ped.med.osaka-u.ac.jp).
References
Ruiz L, Delgado S, Ruas-Madiedo P, Sanchez B, Margolles A (2017) Bifidobacteria and their molecular communication with the immune system. Front Microbiol 8:2345. https://doi.org/10.3389/fmicb.2017.02345
Roy S, Trinchieri G (2017) Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 17:271–285. https://doi.org/10.1038/nrc.2017.13
Sivan A, Corrales L, Hubert N et al (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350:1084–1089. https://doi.org/10.1126/science.aac4255
Tanoue T, Morita S, Plichta DR et al (2019) A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565:600–605. https://doi.org/10.1038/s41586-019-0878-z
Kitagawa K, Oda T, Saito H et al (2017) Development of oral cancer vaccine using recombinant Bifidobacterium displaying Wilms’ tumor 1 protein. Cancer Immunol Immunother 66:787–798. https://doi.org/10.1007/s00262-017-1984-0
Rosenfeld C, Cheever MA, Gaiger A (2003) WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia 17:1301–1312. https://doi.org/10.1038/sj.leu.2402988
Cheever MA, Allison JP, Ferris AS et al (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15:5323–5337. https://doi.org/10.1158/1078-0432.CCR-09-0737
Yokoyama WM (2001) Monoclonal antibody supernatant and ascites fluid production. Curr Protoc Immunol 2:2–6. https://doi.org/10.1002/0471142735.im0206s40
Andrew SM, Titus JA (2001) Purification of immunoglobulin G. Curr Protoc Immunol 2:2–7. https://doi.org/10.1002/0471142735.im0207s21
Laky K, Kruisbeek AM (2016) In Vivo depletion of T Lymphocytes. Curr Protoc Immunol 113(1):4–1. https://doi.org/10.1002/0471142735.im0401s113
Hamanishi J, Mandai M, Abiko K, Matsumura N, Baba T, Yoshioka Y, Kosaka K, Konishi I (2011) The comprehensive assessment of local immune status of ovarian cancer by the clustering of multiple immune factors. Clin Immunol 141:338–347. https://doi.org/10.1016/j.clim.2011.08.013
Oka Y, Udaka K, Tsuboi A, Elisseeva OA, Ogawa H, Aozasa K, Kishimoto T, Sugiyama H (2000) Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol 164:1873–1880. https://doi.org/10.4049/jimmunol.164.4.1873
Nakajima H, Oka Y, Tsuboi A et al (2012) Enhanced tumor immunity of WT1 peptide vaccination by interferon-beta administration. Vaccine 30:722–729. https://doi.org/10.1016/j.vaccine.2011.11.074
Takada T, Matsumoto K, Nomoto K (2004) Development of multi-color FISH method for analysis of seven Bifidobacterium species in human feces. J Microbiol Methods 58:413–421. https://doi.org/10.1016/j.mimet.2004.05.006
Sunde PT, Olsen I, Gobel UB, Theegarten D, Winter S, Debelian GJ, Tronstad L, Moter A (2003) Fluorescence in situ hybridization (FISH) for direct visualization of bacteria in periapical lesions of asymptomatic root-filled teeth. Microbiology 149:1095–1102. https://doi.org/10.1099/mic.0.26077-0 (Reading)
Kayama H, Kohyama M, Okuzaki D et al (2018) Heme ameliorates dextran sodium sulfate-induced colitis through providing intestinal macrophages with noninflammatory profiles. Proc Natl Acad Sci U S A 115:8418–8423. https://doi.org/10.1073/pnas.1808426115
Balic A, Smith KA, Harcus Y, Maizels RM (2009) Dynamics of CD11c(+) dendritic cell subsets in lymph nodes draining the site of intestinal nematode infection. Immunol Lett 127:68–75. https://doi.org/10.1016/j.imlet.2009.09.001
Fink LN, Frokiaer H (2008) Dendritic cells from Peyer’s patches and mesenteric lymph nodes differ from spleen dendritic cells in their response to commensal gut bacteria. Scand J Immunol 68:270–279. https://doi.org/10.1111/j.1365-3083.2008.02136.x
Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W (2018) CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 18:635–647. https://doi.org/10.1038/s41577-018-0044-0
Klinman DM, Barnhart KM, Conover J (1999) CpG motifs as immune adjuvants. Vaccine 17:19–25. https://doi.org/10.1016/s0264-410x(98)00151-0
Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764. https://doi.org/10.1084/jem.20070590
Zitvogel L, Kroemer G (2014) CD103+ dendritic cells producing interleukin-12 in anticancer immunosurveillance. Cancer Cell 26:591–593. https://doi.org/10.1016/j.ccell.2014.10.008
Mohseni AH, Taghinezhad SS, Keyvani H (2020) The first clinical use of a recombinant Lactococcus lactis expressing human papillomavirus type 16 E7 oncogene oral vaccine: a phase I safety and immunogenicity trial in healthy women volunteers. Mol Cancer Ther 19:717–727. https://doi.org/10.1158/1535-7163.MCT-19-0375
Acknowledgements
The authors thank Mr. H. Yachi and K. Kärre, MD, PhD for their kind supply of study material and are grateful to S. Sakima, MD, for valuable discussions.
Funding
NN received research grant from the Japan Society for the Promotion of Science Grants in Aid for Scientific Research (17K16258), and YH received research grant from the same organization (17K10109) and Translational Research Program, Strategic Promotion for Practical Application of Innovative Medical Technology (TR-SPRINT) from the Japan Agency for Medical Research and Development (AMED) under grant numbers 17lm0203036h0001 and 19lm0203091s0101.
Author information
Authors and Affiliations
Contributions
YH, AT, and NN designed the study and reviewed all data. TS and TK designed the vaccines. HN, HK, and RO conducted the pathology. NN, HM, JN, AT, HK, SM, and HN conducted the immunologic assays. NN and HM led the clinical care of the animals. HK provided purified proteins. NN, YH, KO, AT, KT, and FF wrote the paper with all co-authors.
Corresponding author
Ethics declarations
Conflict of interest
TS, TK, and YH are the co-inventors on related vaccine patents. All authors have nothing to declare about this study.
Ethical approval
Our study was approved by Osaka University Animal Experiment Committee. Osaka University’s Safety Management Regulations on Recombinant DNA Experiment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakagawa, N., Hashii, Y., Kayama, H. et al. An oral WT1 protein vaccine composed of WT1-anchored, genetically engineered Bifidobacterium longum allows for intestinal immunity in mice with acute myeloid leukemia. Cancer Immunol Immunother 72, 39–53 (2023). https://doi.org/10.1007/s00262-022-03214-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03214-4