Abstract
Purpose
The most frequent mutation in advanced non-small–cell lung cancer (NSCLC), Kirsten rat-sarcoma viral oncogene (KRAS) is found in 20–25% of these patients’ tumors. While phase III trials on therapies targeting KRAS, especially KRASG12C, are ongoing, the clinical efficacy of anti-programmed death protein-1 (PD-1) or its ligand (PD-L1) against KRAS-mutant NSCLCs remains a topic of debate.
Methods
This meta-analysis examined randomized-trial data comparing first- or second-line anti-PD-(L)1 with or without chemotherapy vs. chemotherapy alone for advanced KRAS-mutant NSCLCs. Outcome measures included overall survival (OS) and progression-free survival (PFS). Analyses were computed using the Cochrane method of collaboration for meta-analyses, with Review Manager software (RevMan version 5.3; Oxford, UK).
Results
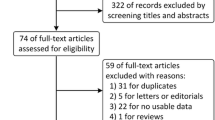
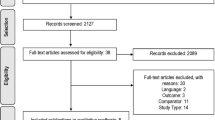
We analyzed 3 first-line trials (IMpower-150, Keynote-189 and Keynote-042) and 3 second-line trials (Oak, Poplar and CheckMate-057) that included 1313 NSCLCs (386 KRAS-mutant and 927 KRAS wild-type tumors). For KRAS-mutant NSCLCs, anti-PD-(L)1 with or without chemotherapy was significantly associated (hazard ratio [95% confidence interval]) with prolonged OS (0.59 [0.49–0.72]; p < 0.00001) and PFS (0.58 [0.43–0.78]; p = 0.0003) compared to chemotherapy alone. OS benefited in both first- and second-line trials. OS for patients with KRAS-mutant NSCLCs was significantly longer than that for those with KRAS wild-type tumors (p = 0.001).
Conclusions
Anti-PD-(L)1 with or without chemotherapy seemed to achieve longer OS and PFS than chemotherapy alone for patients with KRAS-mutant and wild-type KRAS advanced NSCLCs, with an even greater OS benefit for the former.





Similar content being viewed by others
References
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C et al (2018) Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 29(Suppl 4):192–237
El Osta B, Behera M, Kim S, Berry LD, Sica G, Pillai RN et al (2019) Characteristics and outcomes of patients with metastatic kras-mutant lung adenocarcinomas: the lung cancer mutation consortium experience. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 14(5):876–889
Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J et al (2017) Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov juin 7(6):596–609
Dogan S, Shen R, Ang DC, Johnson ML, D’Angelo SP, Paik PK et al (2012) Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res Off J Am Assoc Cancer Res 18(22):6169–6177
Goulding RE, Chenoweth M, Carter GC, Boye ME, Sheffield KM, John WJ et al (2020) KRAS mutation as a prognostic factor and predictive factor in advanced/metastatic non-small cell lung cancer: a systematic literature review and meta-analysis. Cancer Treat Res Commun 24:100200
Agyeman A, Vallejo JJ, Myers A, Blumenthal GM. (2018). Meta-analysis exploring the effect of oncogenic driver mutations on outcome of metastatic non-small cell lung cancer (mNSCLC) patients (pts) treated with immune checkpoint inhibitors (ICI) or docetaxel (doc). J Clin Oncol. 36(15_suppl): 9029‑9029.
Torralvo J, Friedlaender A, Achard V, Addeo A (2019) The activity of immune checkpoint inhibition in kras mutated non-small cell lung cancer: a single centre experience. Cancer Genomics Proteomics 16(6):577–582
Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V et al (2018) Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 4(2):210–216
Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. (2020). Atezolizumab Plus Chemotherapy for First-Line Treatment of Non-Squamous Non-Small Cell Lung Cancer: Results From the Randomized Phase III IMpower132 Trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer.
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ et al (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol juill 20(7):924–937
Herbst RS, Lopes G, Kowalski DM, Kasahara K, Wu Y-L, Castro GD et al (2019) LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann Oncol 30:xi63–xi64
Gadgeel S, Rodriguez-Abreu D, Felip E, Esteban E, Speranza G, Reck M et al (2019) LBA5–KRAS mutational status and efficacy in KEYNOTE-189: Pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol 30:xi64–xi65
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet Lond Engl 387(10030):1837–1846
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet Lond Engl. 389(10066):255–265
Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L et al (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol Off J Eur Soc Med Oncol 30(8):1321–1328
Ng TL, Liu Y, Dimou A, Patil T, Aisner DL, Dong Z et al (2019) Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer 125(7):1038–1049
Scheel AH, Ansén S, Schultheis AM, Scheffler M, Fischer RN, Michels S et al (2016) PD-L1 expression in non-small cell lung cancer: correlations with genetic alterations. Oncoimmunology 5(5):e1131379
Jeanson A, Tomasini P, Souquet-Bressand M, Brandone N, Boucekine M, Grangeon M et al (2019) Efficacy of Immune checkpoint inhibitors in kras-mutant non-small cell lung cancer (NSCLC). J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 14(6):1095–1101
Aredo JV, Padda SK, Kunder CA, Han SS, Neal JW, Shrager JB et al (2019) Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer Amst Neth juill 133:144–150
Arbour KC, Rizvi H, Plodkowski AJ, Hellmann MD, Knezevic A, Heller G, et al. Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 8 févr;
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI et al (2020) KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med 383(13):1207–1217
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Landre, T., Justeau, G., Assié, JB. et al. Anti-PD-(L)1 for KRAS-mutant advanced non-small–cell lung cancers: a meta-analysis of randomized–controlled trials. Cancer Immunol Immunother 71, 719–726 (2022). https://doi.org/10.1007/s00262-021-03031-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-03031-1